Goal is to shift glucose testing away from medical laboratories and make it easier for diabetics to do their own testing, while capturing glucose test results in patient records
Because of the tremendous volume of glucose tests performed daily throughout the world, many companies are developing non-invasive methods for glucose testing. Their goal is a patient-friendly technology that does not require a needle stick or venipuncture and may even eliminate the need to send specimens to a medical laboratory.
What is intriguing about these initiatives is that, in their final form, they may create a flow of useful diagnostic data reported to clinical laboratories in real time. This would create the opportunity for pathologists and lab scientists to consult with the patients’ physicians, while archiving this test result data in the laboratory information system (LIS).
These glucose monitoring methods would also ensure that a complete longitudinal record of patient tests results is available to all the physicians practicing in an accountable care organization (ACO), medical home, or hospital.
Number of Patients Diagnosed with Type 2 Diabetes Exploding
There is rocket fuel propelling companies’ interest in developing improved glucose tests. It is the explosive increase in the number of individuals with Type 2 diabetes. Almost 30 million Americans are diabetics, and it is estimated that more than 70 million Americans are pre-diabetic. Pathologists and clinical laboratory managers should keep these facts in mind as they watch how biotech companies attempt to market new methods for glucose testing.
There is wide variety in the technology approaches being taken by companies that want to bring the next big thing in glucose testing to market. These include glucose testing devices that are wearable or that are point-of-care (POCT).
Companies are designing these as multi-function devices that noninvasively monitor glucose, dehydration levels, and pulse rate.
Some devices use a light-sensing technology. Other devices are breathalyzers that monitor glucose and can also indicate presence of disease and other conditions, such as lung cancer, diabetes, and high cholesterol.
Lucrative Market for Noninvasive, Point-of-Care Glucose Testing Devices
Certainly pathologists and medical laboratory professionals will be watching closely to see if a breathalyzer for monitoring glucose can produce immediate results that are accurate and reproducible, at a cost that is equal to or lower than glucose assays currently performed by clinical laboratories. If such a device were to be developed, it would have a ready market in hospitals and physician offices.
Similarly, were vendors to develop a wearable glucose monitor that is accurate, does not require a finger stick and offers comparable or lower costs than today’s generation of glucose test kits and supplies, such a device would likely enjoy strong consumer acceptance, along with favorable coverage decisions by private insurance plans.
Wearable Glucose and Biometric Monitors Use Light-Sensing Technology
Several companies are developing wearable, watch-like devices. These are capable of tracking certain biometrics and monitoring glucose. The devices commonly use changing patterns of scattered light—or the so-called “speckle pattern” effect—in which grainy interference patterns are produced on images.
This happens when laser light reflects on an uneven surface or scatters on an opaque material, according to a press release issued by The Optical Society (OSA). OSA’s journal, Biomedical Optics Express (BOE) published papers recently describing these types of glucose testing devices.
This technique is based on tracking of temporal changes of reflected secondary speckles produced in the wrist when being illuminated by a laser beam, explained biomedical engineer Mahsa Nemati, a graduate student in the Optics Research Group at the Delft University of Technology in the Netherlands. When the material that is scattering the light is moving—say, in the case of blood flowing through the circulatory system—“the speckle pattern changes with changes in the flow,” she said.
Nemati was the lead author of a paper describing research that produced a wearable device that tracks pulse rate. This research paper was published by BOE on July 1, 2014.
Glucose Testing Research at Israel’s Bar-Illan University
Zeev Zalevsky, Ph.D., a bioengineer and Professor at Bar-Ilan University, authored the paper that presented Israel’s Bar-Illan University research to create the first watch-like, wearable, noninvasive glucose-monitoring device. This device can also simultaneously or independently estimate a user’s level of dehydration during activity. This paper was published by BOE June 1, 2014.

At Israel’s Bar-Ilan University, a research team led by Zeev Zallevsky, Ph.D. (pictured above), has developed a non-invasive glucose measuring device that is worn like a wristwatch. The device can also measure the user’s dehydration during activity. (Photo copyright The Society of Electrical and Electronics Engineers in Israel.)
This device consists of a laser that generates a wavefront of light to illuminate a patch of skin on the wrist near an artery, and a camera that measures changes over time in the light backscattered off the skin. Unlike other chemicals present in the blood, glucose exhibits a “Faraday effect,” noted the OSA press release. In the presence of an external magnetic field generated by the attached magnet, the glucose molecule alters the polarization of the wavefront and thus influences the resulting speckle patterns. These changing patterns provide a direct measurement of the glucose concentration.
“The importance of magnet is it allows interaction only with glucose and not other materials, because of the high Verdet constant of the glucose molecule in contrast to molecules of other materials in our blood stream,” wrote Zalevsky in the BOE paper.
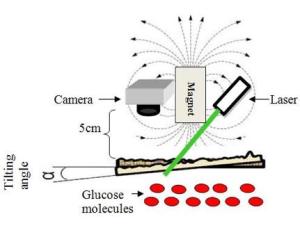
The schematic diagram above shows how the new system can be used to measure a person’s glucose levels noninvasively. A laser generates a wavefront of light to illuminate a patch of skin on the wrist near an artery. The camera measures changes in light scattered off the skin, which creates a “Faraday” effect. This means that in presence of a magnetic field, which is generated by the attached magnet, the glucose molecule alters the polarization of the wavefront to influence the resulting speckle pattern. (Photo copyright Biomedical Optics Express)
The same device without the attached magnet estimates the relative dehydration level of the user as it changes during activity. Muscle weakness is a primary indicator of mild to moderate dehydration, said Zalevsky, explaining that the device detects changes in hydration by strength of optical signals, which are altered as muscle weakens.
“This is the first step toward noninvasive, continuous in vivo measurement of glucose based on sensing an effect that is directly related to glucose concentration,” Zalevsky observed in the OSA press release.
The team expects a commercial version of the device, to be available in the marketplace within two to three years. In the meantime, Zalevsky and his colleagues are working to reduce the margin of error in the device’s readings, which were within a range of 15% deviation from a medical glucometer device for 96% of the study’s measurements.
Wearable Pulse Tracker is Accurately Detects Heart Rate in Moving User
Nemati’s paper described research proving the wearable pulse tracker accurately detects heart rate in a moving subject. “This paper shows, for the first time, that speckle pattern generated from a flowing liquid can give us the pulsation properties of the flow, in spite of motion-induced artifacts,” she said.
“Sophisticated optics is not necessary to implement this, so the costs for devices can be kept low,” Nemati noted.

In a) is the schematic the experimental setup of the pulse monitor, and b) a photo of the actual experimental setup. The researchers used simulated heartbeats generated in milk and measurements performed on the finger of a volunteer to test the accuracy of speckle changes in measuring flow pulsations—that is, the heart rate. The pulse tracker accurately detected heart rate even when the light source used to create the speckle pattern was moving, as would be the case with a wearable biometric sensor. (Photo copyright Biomedical Optics Express)
Breathalyzer’s Nanosensor Detects Disease Biomarkers in Exhaled Breath
Nanotechnology was used to create a breathalyzer to diagnose diseases and disorders, well as noninvasively monitor glucose levels in diabetics.
New York’s Stony Brook University Associate Professor Perena Gouma, Ph.D., led a team of researchers that developed nanosensor chip that serves as the “brain” of a breathalyzer that can detect biomarkers for disease and other health conditions, including lung cancer, diabetes and high cholesterol. The sensor is coated with tiny nanowires that can detect minute amounts of chemical compounds in exhaled breath that signal disease.
Gouma explained in an interview with PBS Newshour, “Each nanowire can capture a particular chemical, a particular compound.” She noted, for example, that ammonia is a marker that for a particular kidney problem and acetone indicates diabetes.
She described research leading to this hand-held breathalyzer in a paper published by IEEE’s Sensors Journal in 2010.
The device is currently in clinical trials and could one day be widely available, noted PBS NewsHour Science Correspondent Miles O’Brien.
Glucose Breathalyzer Uses Nano-films and Acetone-Sensitive Polymers
Western New England University (WNE) researchers also announced another breathalyzer that uses nanotechnology to noninvasively detect blood-glucose levels in the breath of diabetics.
The researchers unveiled this technology at the 2013 American Association of Pharmaceutical Scientists (AAPS) Annual Meeting and Exposition in San Antonio, Texas, according to a press release issued by AAPS.
Ronnie Priefer, Ph.D., a Professor of Medical Chemistry at WNE in Springfield, Massachusetts, created the multilayer technology using nanometer-thick films consisting of two polymers that react with acetone. This film crosslinks the polymers and alters the physicochemical nature of the film to provide quantification of acetone, and thus glucose levels, noted the AAPS press release. (Remember: acetone is an indicator of diabetes.)
Breathalyzers Designed for Diabetes Monitoring
“Breathalyzers are a growing field of study because of their potential to have significant positive impact on patients’ quality of life and compliance with diabetes monitoring,” said Priefier.
“What makes our technology different is that it only accounts for acetone and doesn’t react with other components in the breath.”
WNE medical clinics will perform controlled testing of the device with patients, beginning in late 2014 or early 2015. Priefer said that testing will compare the glucose readings of the breathalyzer, finger prick, and actual glucose levels from blood drawn by a phlebotomist and tested in a clinical laboratory.
Large and Fast-Growing Market for Non-Invasive Glucose Testing
Two things motivate this research into alternative forms of glucose testing for Type 2 diabetes patients. One motivation is to design a reliable testing methodology that is not invasive and does not require a needle stick or venipuncture. This would be patient-friendly and would find a ready market.
The second motivation is the huge volume of glucose testing that must be performed in order to help diabetes patients monitor their health. Not only is this a lucrative market today, but it will be one of the fastest-growing sectors of clinical laboratory testing for years to come, given the current population demographics and incidence of Type 2 diabetes.
—By Patricia Kirk
Related Information:
Light used to non-invasively monitor glucose, dehydration, pulse in new biometric watches
Towards a Wearable Non-invasive Blood Glucose Monitoring Device
Apple Creating an iWatch That Could Monitor Glucose?!
Breathalyzer technology detects acetone levels to monitor blood glucose in diabetics
Breathalyzer Detects Diseases From Diabetes to Cancer
Nanosensor and Breath Analyzer for Ammonia Detection in Exhaled Human Breath




my questions were directed at the post above regarding the laser device in use since 2008.
is 90% accuracy enough to rely on for treatment?? is this device affordable?? where can i get one?? can i be kept informed of all the developments of this beast??
It is possible to use it also for little boy with type 1 diabete?
It is possible to buy it already
thanks
seeking insight on your plans thank you leo
Jose,
I am interested in your post about the laser glucose monitor. How can I contact you?
Thank you in advance.
I have a portable laser glucose monitor that was invented in 2008 and its 90% accurate. This device has been in used since 2008 at major hospital in Europe. We need less then 5% to meet FDA approval with no restrictions. The inventor and investor has decide that now is the right moment to sell the device. Want to hear your comments