Many products that medical laboratories use will be covered under the new UDI system
You’re reading it here first! UPCs—universal product codes—are coming soon to the medical laboratory analyzers and other products that your clinical laboratory purchases. Under a proposed rule published by the Food and Drug Administration (FDA), medical devices will soon have UDIs—universal device identifiers.
You know about UPCs. Those are the ubiquitous “universal product codes” that are found on literally every retail product. UPCs make scanning at the cash register possible. Now a similar system is coming to medical devices, including the lab analyzers, reagents, and other products used by medical laboratories and pathology.
Unique Device Identifiers (UDI) Are Like Universal Product Codes (UPC)
In July, the Food and Drug Administration (FDA) finally published its proposed rule on unique device identifiers (UDI) in the Federal register. The public can comment on this proposed rule through November 7, 2012.
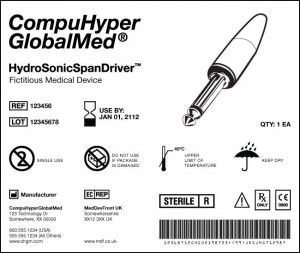
On its website, the Food and Drug Administration (FDA) provides this example of how a universal device identifier (UDI) would be incorporated on a medical device label. As shown above, such a label would contain information about the product name, its expiration date, reference and lot numbers, manufacturer information, bar code, details about the item, and an illustration of the item. Medical devices, including clinical laboratory analyzers and related products, would have unique UDIs, once implementation of the proposed rule becomes effective. (Image by the FDA.)
The government’s new product identifier system is an effort to improve patient safety and efficiency. The initiative moves healthcare toward the same kind of Universal Product Code (UPC) system that retail products have. This is important for pathologists and clinical laboratory managers because many products that medical laboratories use will be covered under the system. These include in vitro diagnostic testing systems, which are considered medical devices.
According to a recent story published in ModernHealthcare.com (MH), the new system is expected to help achieve three important goals:
- improve recalls and adverse event reporting;
- reduce medical errors; and,
- establish consistent standards for device data entry into electronic health records (EHR) and clinical information systems.
The FDA will benefit from UDIs. The agency expects the system to enhance its pre- and post-market analysis of devices, as noted on its website.
“Patients today face a significant risk that a recalled or defective medical device could be used in their treatment because of the lack of consistent and reliable methods for a hospital or physician to quickly and reliably identify such devices,” wrote the authors of a position paper. This paper was published by Premier, Inc..
Premier is a hospital-owned group purchasing organization (GPO) that has advocated for the proposed rule. It consists of an alliance of more than 2,400 U.S. hospitals and roughly 70,000 other health care sites.
UDI System Will Increase Efficiency at Hospitals
“The benefits come about from electronic data capture and the movement and exchange of that information and then the visibility that [information] provides,” stated Jay Crowley, Senior Adviser for Patient Safety at the FDA’s Center for Devices and Radiological Health, in the MH story. Mercy Health System in St. Louis provides an example.
Mercy began putting new scanning technology on its nursing floors within the last couple of years, MH reported. Because of these scanners, nurses can now record a patient’s use of a medical product by scanning its bar code. Not only does the scan sends a notice to refill the product, but it also transfers the data to an EHR and bills the patient!
UDIs Can Improve Patient Safety in Hospitals and Other Care
Settings “The patient-safety benefits and business-efficiency benefits will significantly outweigh any kind of incremental costs that we incur,” stated Gene Kirtser, President and Chief Executive Officer of Resource Optimization & Innovation (ROi), in the MH story. ROi is Mercy’s GPO.
Under the proposed rule, the UDI markings will be phased in gradually over a ten-year period, a story in Nature reported. The riskiest devices will carry the codes within a year after the final rule is published. The cost for implementing the system is estimated at more than $500 million over the next decade.
Clearly, providers that are early adopters of health technology will be the early adopters of standards identification systems, observed Siobhan O’Bara, Vice President for Healthcare for GS1 U.S.. GS1 is an international non-profit organization that works to standardize supply chain systems. (See Dark Daily, “Hospitals, Clinical Labs, and Pathology Groups Will Soon Adopt Global Location Systems”.)
“Those healthcare systems that have the infrastructure in place will be able to much more quickly take advantage of and accrue the benefits of a standardized identification system,” FDA’s Crowley noted.
UDI System Is Essential to Maximizing Value of EMRs
The FDA does not have the authority to require providers to use the system. That means the initiative depends on the voluntary participation of hospitals and other providers, including clinical laboratories.
The UDI program is largely infrastructure-dependent. “The [EHR] is a [huge] piece of this,” Crowley pointed out in MH. “Those healthcare systems that have the infrastructure in place will be able to much more quickly take advantage of, and accrue the benefits of, a standardized identification system.”
Pathology groups and clinical laboratory managers will want to assure that their medical laboratories are integrating global location numbers and unique device identifiers into their information systems as soon as practicable.
—Pamela Scherer McLeod
Related Information:
Benefits of Unique Device Identification
New tracking system proposed to help recall faulty devices
Medical Devices: FDA Should Enhance Its Oversight of Recalls
Dark Daily, “Hospitals, Clinical Labs, and Pathology Groups Will Soon Adopt Global Location Systems”



