Trend is toward increased use of automation, particularly modular and task-targeted solutions, by medical laboratories across the globe
![]()
Worldwide, growing numbers of clinical pathology laboratories and medical laboratories are purchasing total laboratory automation (TLA) systems. This is evidence that the latest generation of laboratory automation products are more robust in use compared to prior years and can be purchased at a price that produces an acceptable return on investment (ROI).
However, the acceptance of total laboratory automation in clinical pathology laboratories is not universal. Only in selected countries have significant numbers of medical laboratories embraced laboratory automation as a way to improve productivity, reduce errors, and boost quality.
Not surprisingly, Japan may be considered the hotbed of total laboratory automation. It was back in the 1980s when four pathologists at different medical schools in Japan began to apply automation to work processes involved in the pre-analytical, analytical, and post-analytical steps of medical laboratory testing. A number of in vitro diagnostics (IVD) companies in Japan licensed these inventions or developed automation technology in collaboration with these and other pioneering pathologists in that country.
Also not surprising is the fact that Japan is home to the world’s longest ongoing conference on clinical pathology laboratory automation. That is the Laboratory Automation and Robotics Conference (also known as the Cherry Blossom Symposium), which takes place every other year. In April, 2010, the most recent conference was conducted in Yokohama, Japan.
In his presentation at the last Cherry Blossom Symposium about the past, present, and future of total laboratory automation, Motoharu Shindo, who manages in the Medical Systems Sales and Marketing Division at Hitachi High Technologies Corporation, based in Tokyo, Japan, provided an overview of the market for clinical laboratory automation. He discussed each generation of laboratory automation, noting that Hitachi began selling its first generation automated chemistry solution in 1970 and was shipping that system to Europe by 1978.
Hitachi’s first generation lab automation system was introduced in 1989. It was a centrifuge and aliquoter connected to a chemistry analyzer. In 1993, Hitachi’s second generation lab automation reached the market. This automated system connected a number of stand-alone instruments, both pre-analytic and analytic, with a conveyor line that was powered by compressed air.
By 1999, Hitachi’s third generation laboratory automation system was in the marketplace. This was a total system approach that was specifically designed to support modular analyzers and subsystems. In his presentation, Shindo said that, from its introduction in 1999 through its latest enhancements in 2010, Hitachi’s third generation TLA has been continuously improved and upgraded.
Further, he noted that the performance emphasis of this generation of TLA was two-fold. First, the TLA system is designed to support improved work processes in the clinical laboratory. Second, the TLA system is designed to deliver a higher performance reliability than that of the laboratory analyzers which are connected to the automation track.
What may be of most interest to Dark Daily readers, however, are the statistics provided by Shindo that demonstrate marketplace acceptance of total laboratory automation. Three charts are reproduced below from Shindo’s presentation at the Automation and Robotics Conference in Yokohama last April, with his permission.
A. Total Number of Hitachi 3rd Generation TLA Installations Worldwide

Slide A: This table presents the number of third-generation Hitachi TLA installations worldwide, beginning in 1999 and running through 2010 (including projects under preparation). Pathologists and laboratory directors should note the exponential nature of this curve, which begins to significantly ramp up in 2002, when Hitachi introduces its TLA in Europe (blue color). Uptake by the United States (red color), where sales started about the same time, are not as numerous. (Courtesy of Hitachi High Technologies Corporation ©)
B. Worldwide Distribution of Hitachi TLA Installations

Slide B: This slide offers three interesting observations. First, Japan, a nation of 128 million people, has 242 Hitachi TLA installations. Second, European labs seemed to have embraced TLA, with 292 total Hitachi TLA installations found in an area with a population in the European Union of approximately 500 million. Third, the United States, with a population of 310 million (2.4 times that of Japan), has just 134 Hitachi TLA installations. (Courtesy of Hitachi High Technologies Corporation ©)
C. European Distribution of Hitachi TLA Installations
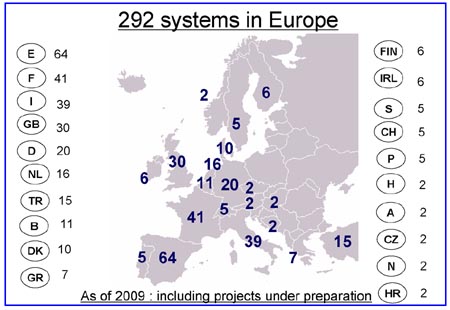
Slide C: The surprise in this slide may be the acceptance of TLA by Spanish laboratories, which support 64 TLA installations, or 22% of all the Hitachi TLA installations in Europe. France is second with 41 TLA installations, followed by Italy and the United Kingdom at 39 and 30 Hitachi TLA installations, respectively. (Courtesy of Hitachi High Technologies Corporation ©)
It is important for pathologists and clinical laboratory managers to keep in mind that these worldwide statistics represent only Hitachi TLA systems. There are a number other in vitro diagnostics manufacturers which sell clinical laboratory total laboratory automation systems. Of course, all the globe’s biggest IVD companies offer total laboratory automation systems, including Roche Diagnostics, Siemens Diagnostics, Abbott Diagnostics, and Beckman Coulter Inc. (NYSE:BEC).
As well, there are Asian companies which have competitive TLA systems in use in Japan and other countries. These companies are unfamiliar to pathologists and clinical laboratory managers in the United States because either they do not sell their products in this country, or their equipment systems are sold in this country under license by one of the major IVD firms mentioned above.
What is important about Hitachi’s presentation at the Automation and Robotics Conference in Yokohama, Japan, is that it provides a window into the market acceptance of TLA solutions by clinical laboratories. In just 11 years, Hitachi’s TLA system alone has grown from a couple of laboratory sites to an installed base of 722 clinical pathology laboratories worldwide.
Assume that there are comparable rates of growth for the TLA solutions offered by Hitachi’s competitors during this same time period. Collectively, the total number of laboratories now using TLA worldwide has soared over the past decade.
That is compelling market evidence that the current generation of TLA solutions is delivering value to laboratories. Second, this widespread adoption of TLA shows how laboratories, faced with declining reimbursement, are willing to invest substantial capital to deploy TLA as a way of making their laboratories more efficient.
Related Information:
Web site of the 7th International Conference of Clinical Laboratory Automation and Robotics
Yokohama’s Cherry Blossom Symposium Showcases Clinical Lab Automation Breakthroughs
Experts in Clinical Pathology Laboratory Automation to Gather in Yokohama



