Advances in use of probe-based Confocal Laser Endomicroscopy (pCLE) could mean that GIs refer fewer specimens to clinical pathology laboratories
Gastroenterologists are beginning to use what is being called the “world’s smallest microscope” to view tissue in situ and diagnose disease. It is a technology innovation that will have important ramifications for the anatomic pathology profession because this new system is designed to allow physicians to microscopically examine a patient’s GI tissue at the cellular level in its natural environment.
The product is entering clinical use in the United States. It is called Cellvizio and is manufactured by Mauna Kea Technologies (MKEA), a French company with offices in Newtown, Pennsylvania. Cellvizio is a miniature microscope that, once inserted into the GI tract, enables the physician to select cells for microscopic examination in order to make a more immediate decision regarding a diagnosis, as well as treatment.
The Cellvizio was cleared by the Food and Drug Administration (FDA) with two 510K approvals in September 2005 and August 2006 and received CE mark in Europe in January of 2006. The company’s second-generation system, the Cellvizio 100 series was cleared in Europe and US in 2011. Although this micro-miniature microscope (with over 40 world-renowned peer-reviewed clinical data) is allowing gastroenterologists to evaluate individual cells and identify abnormal or malignant tissue, there will still be a need to collect a tissue biopsy for analysis of its DNA, RNA, and/or proteins. The complexity of those genetic tests and molecular diagnostics assays means that most of these tissue referrals will continue to be sent to pathologists.
One contributing technology that went into this micro-miniature microscope is a high-density digital chip capable of performing several functions within a tiny module.
Medical Centers Already Use This New Micro-Mini Microscope System
As many as 50 Cellvizio systems may be in use currently in the United States. In clinical settings, physicians use the system to examine live, moving gastrointestinal (GI) and biliary tissues in patients.
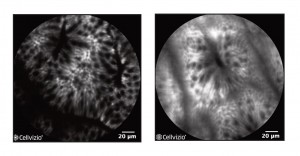
Here are photos of the physician can see when he or she uses the Cellvizio system to view the intestinal tract. The photo at left is of colon hyperplasia. The photo at right is a normal colon tissue. The "mini-microscope" capability of this system allows the physician to see individual cells. (Photo provided by Mauna Kea Technologies, Inc.)
In Philadelphia, physicians at one of the area’s biomedical research institutes have had success with the Cellvizio focal probe. One of them is Bob Etemad, M.D., gastroenterologist at Lankenau Medical Center and Medical Director of Endoscopy at the Main Line Health System.
“Until now, if we found areas that appeared abnormal on endoscopy during one of our endoscopic procedures, we would have to [biopsy the tissue and] send it to a laboratory for analysis—which can take up to a week—to see what it looked like under the microscope,” stated Etemad, in a story published by Marketwatch.com.
“This sometimes is a problem when the biopsies do not confirm our suspicions,” continued Etemad. “We may then need to rebiopsy with an additional procedure. Also, the eye is not as sensitive to detect some subtle but dangerous precancerous changes.”
Etemad and his team now have a tool that helps them better identify the dangerous tissue during the initial diagnostic exam. This allows them to remove this tissue the same day, and then go back to ensure all of the diseased tissue was removed.
The Lankenau team is one of the first in the United States to use this new approach. It will be applied to gastrointestinal cancers and other GI diseases, including those of the colon, bile duct, pancreas, and esophagus. Etemad recently treated a patient originally diagnosed with Barrett’s esophagus, an abnormal precancerous change in the lower esophagus. Originally, this patient was told by her physician that her esophagus needed to be removed. The patient then got a second opinion from Dr. Etemad and learned that invasive surgery was not necessary. After three minimally invasive endoscopy procedures, she is cancer-free.
Essentially, the Cellivizio system is a focal probe threaded through a traditional endoscope. It is just one more example of how new technology breakthroughs are creating products that challenge the status quo in surgical pathology.
Possibility of Fewer GI Tissue Biopsy Referrals to Pathology Laboratories
Because gastroenterology groups generate large numbers of tissue specimens that are typically referred to pathologists for processing and diagnosis, a system such as Cellvizio has the potential to reduce the number of biopsies collected by GIs and referred to pathologist for diagnosis.
On the other hand, even if this micro-miniature microscope can allow gastroenterologists to evaluate individual cells and identify abnormal or malignant tissue, there will still be a need to collect a tissue biopsy for analysis of its DNA, RNA, and/or proteins. The complexity of those genetic tests and molecular diagnostics assays means that most of these tissue referrals will continue to be sent to pathologists.
At the same time it is important to recognize that this Cellvizo system has the potential to pull more testing away from the traditional anatomic pathology laboratory. In looking at this system, Michael J. Cima, Ph.D., at the Massachusetts Institute of Technology, recognized that possibility when he stated that, by using these types of systems to look at tissue in situ, “we are going to bring the laboratory into the patient.”
Related Information:
Mauna Kea Technologies: Section 7: Premarket Notification 510(k) Summary



