New mHealth devices open the door for clinical laboratories and pathologists to offer continuous monitoring services to patients which incorporate the patient’s self-test results with that patient’s cumulative medical lab test data
In today’s age of mobile computing, healthcare applications are hot. Now comes news that a Swiss company has launched what it calls the world’s first medical smartphone! Of note to clinical laboratory managers and pathologists is the fact that this medical smartphone is designed to capture and analyze several health measures that are often the subject of medical laboratory clinical laboratory tests, including blood gases and blood glucose.
This medical smartphone is manufactured by LifeWatch AG (LIFE:SW), based in Neuhausen am Rheinfall, Switzerland. The LifeWatch V lets users self-test their health.
“The…smartphone allows [users] to self-operate a wide range of highly valuable embedded medical sensors, wellness-related applications, cloud-based services, and 24/7 call center support,” the company stated in a news release. The device operates on an Android operating system.
Medical Smartphone Performs Seven Different Tests
The innovative smartphone enables users to capture, collect, and analyze their health and medical measurements anywhere, anytime, the release stated. The features include:
• ECG;
• body temperature;
• blood glucose;
• heart rate;
• blood oxygen saturation;
• body fat percentage; and
• stress levels (based on heart rate variability).

Billed as the world’s first medical smartphone, the LifeWatch V built-in sensors for monitoring heart rate, pulmonary function, blood oxygen saturation, blood sugar levels, body temperature, and more. The device could give innovative clinical laboratories a new opportunity to continuously monitor an individual’s incoming measurements and provide clinical pathology consultations. (Photo by LifeWatch AG.)
Users of this medical smartphone can share test results and historical user data with doctors, family or others through e-mail or text message, noted a story published by medGadget. Each test is presented as an application. Users can also plan their diets and get detailed reminders about their medications.
“[The medical smartphone] It is first of all intended for health-conscious consumers who have already recovered from, or are suffering from a chronic medical condition,” stated Yacov Geva, D.B.A., Chairman and CEO of LifeWatch, in a story.
The smartphone has numerous consumer-friendly benefits. It will enable patients to consolidate ongoing medical laboratory tests and diagnostics in one convenient place. It will also make traveling easier for people monitoring conditions, such as diabetes. Geva also pointed out that it will be particularly useful for parents monitoring a child’s health data.
Lifewatch’s medical smartphone will cost between $500 and $700, according to a story in The Jewish Press.
“We believe that we will obtain EU CE Mark certification by the end of this year and US Food and Drug Administration (FDA) approval the following year,” Geva stated. “It is already possible to market the device in Israel.”
A Device that Transforms Any Smartphone into a Hospital-Grade ECG
Also in the realm of mHealth and mobile devices adapted for healthcare purposes is a device that allows individuals to perform their own ECGs. Availability of this device was announced in June, by Rehovot, Israel-based SHL TeleMedicine Ltd. (SHL) (SHLTN:SW). At that time, the company had received FDA approval for marketing this innovative device. SHL Telemedicine had earlier received approval in the EU, according to a company press release.
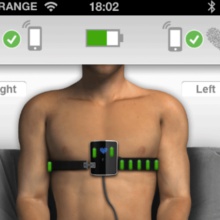
Another device that fits the definition of mHealth is the Smartheart, manufactured by SHL Telemedicine. Smartheart allows smartphone users to perform hospital-grade ECGs and transmit the data in real time. (Graphic by SHL Telemedicine.)
Called Smartheart, “the device transforms any smartphone into a hospital-grade ECG that allows the user to perform a quick and simple test, equal in quality to a professional hospital ECG examination,” stated Erez Alroy, SHL co-CEO. He was quoted in a story published by Globes. “We believe the Smartheart is a technological breakthrough, positioning SHL at the forefront of the mHealth market, opening up access to new target audiences, both in existing and new markets.”
The Smartheart device hooks around the user’s chest. It can transmit results in real time to a physician, personal health record, or hospital. The user can view results at any time on the smartphone through secured access.
Estimated cost for the Smartheart is US$499, according to a story published at phoneArena.com.
Are Medical Smartphones an Opportunity For Clinical Laboratories?
Given such innovations, is it such a stretch to imagine that medical laboratories and pathologists might develop a real-time clinical laboratory testing service that utilizes inbound data feeds from similar devices? In conjunction with the patient’s cumulative lab test result record, innovative mHealth devices might enable the clinical laboratory pathologist to identify changes in smartphone-generated point-of-care lab test data and notify both patient and physician.
—Pamela Scherer McLeod
Related Information:
LifeWatch launches world’s first medical smartphone
LifeWatch V: Android-based Healthcare Smartphone Packed with Medical Sensors
Israeli Medical Smartphone Spreading Freedom, Happiness, Around the World




This is a great innovation and a step in the right direction. I am working with hundreds of labs through Informedika and I think an addition of such revolutionary device to the clinical environment and toolkit, especially for the outpatient care would be tremendous. What would really propel this device adoption rates is the correlation of the device’s feeds with the rest of the diagnostic and pathological (including genetic) data. Would love to have this device’s feeds merged with informedika.com diagnostic feeds, so that it can find its way into physicians’ office FAST!