Complying with this standard will help reduce errors by improving specimen handling and tracking, and also reduce lab costs by boosting accuracy and quality.
Clinical laboratories have about nine months to comply with a new standard for bar code labels. The deadline for compliance to AUTO12-A, Specimen Labels: Content and Location, Fonts, and Label Orientation, is April 29, 2014.
While compliance next year will not affect a lab’s accreditation, in the coming years accrediting bodies, such as The Joint Commission and the College of American Pathologists are expected to require medical laboratories to comply with this bar code label standard.
Clinical Laboratory Professionals Developed Bar Code Label Standard
AUTO12-A, the standard for bar code specimen labels, was developed by advisors from the clinical laboratory industry and is published by the Clinical and Laboratory Standards Institute (CLSI) in Wayne, Pennsylvania. Labs that have already complied with this standard report that compliance has significantly improved specimen tracking.
They also report reduced identification errors and increased productivity, according to Charles D. Hawker, Ph.D., MBA, FACB, the Scientific Director, Automation and Special Projects, for ARUP Laboratories in Salt Lake City, Utah.
Hawker noted that those labs yet to implement the new bar code specimen label will need to hustle to meet the looming deadline.
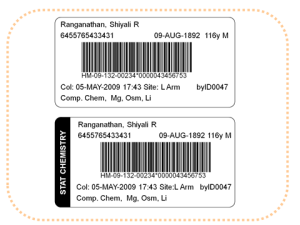
Pictured here are samples of how the clinical laboratory test specimen label looks when produced according to CLSI standard AUTO12-A. The bottom label shows how a label would be prepared for a STAT test.
“If labs don’t act now, they may need to engage in considerable last-minute activity to comply by April 29, 2014,” said Hawker, who chaired the CLSI committee that prepared the standard.
“In December 2012, The Joint Commission, which inspects all hospitals in the United States, reported that it intended to list the bar code label standard as a reference in its prepublication requirements document,” he noted.
“The bar code label standard also will be studied by the College of American Pathologists (CAP) next year (2014) or in 2015, with a goal of including it in the checklist.”
Medical Laboratories Can Benefit from Adopting Bar Code Label Standard
As reported in The Dark Report (See “Standard Bar Code Labels Can Reduce Lab Errors, TDR, May 6, 2013), The adoption and use of the standardized bar code label requires a small up-front investment, but then becomes “the gift that goes on giving.” This investment provides a return in cost savings from a reduction in errors, a factor that is particularly important when lab budgets are shrinking.
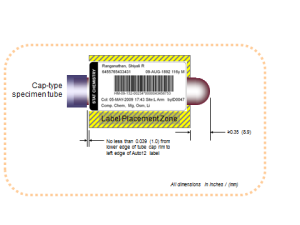
In the image above, the standard bar code specimen label is shown as it should be placed on a cap-type specimen tube. This placement is compliant with the CLSI standard AUTO12-A, which is designed for use by clinical laboratories.
Although the CSLI effort to standardize bar code labels was proposed in 2005, it was not adopted until 2011. That was the year when CLSI issued the approved version of the standard, Hawker said.
“This standard was needed because misidentified or mislabeled specimens happen in laboratories with a greater frequency than what would represent good patient care,” he commented. “A variety of labels also impedes the ability of labs to process specimens quickly.”
Considered an expert on total laboratory automation, Hawker has published several peer-reviewed studies demonstrating that using non-standardized bar code labels on patient specimens contribute to a surprisingly high rate of errors. One study, “Bar Codes May Have Poorer Error Rates Than Commonly Believed,” was published in Clinical Chemistry (CC 56:10–1513–1514, 2010) in 2010.
Studies Show Mislabeling Error Rates in Labs Range from 0.1% to 5%
“Published references cited in the CLSI standard show mislabeling error rates in the United States range from 0.1% to 5%,” commented Hawker, who is an adjunct professor of pathology at the University of Utah School of Medicine.
“Among the published references cited, one report revealed an error rate of nearly 1 per 1,000 in 147 participating clinical laboratories. Another report in the cited sources indicated a mislabeled error rate of 1.12% on blood bank specimens. This latter value is astoundingly high!”
Clinical laboratory managers know that misidentified specimens raise lab costs significantly. In 2005, the average cost of a misidentified specimen was $712, according to one report. “This amount did not include immeasurable costs, such as patient anxiety and discomfort, and the delays in diagnosis and treatment,” Hawker commented. “It also does not include the consequential costs, such as those for any ensuing litigation for actual incurred patient harm.”
Cost of Mislabeled Lab Specimen Estimated to be $712 Per Mislabel
A conservative estimate for the total cost of mislabeled specimens is about $280,000 per million specimens tested, Hawker said. “This estimate comes from multiplying the average cost of $712 per mislabel incident times a low published incidence of mislabeled specimens of 0.039%, according to a one report,” he explained, but noted that this estimate does not include litigation or settlement expenses.
Another source of unmeasured costs comes when laboratory employees need to stop production to find patient names in different locations on nonstandard barcode labels, Hawker explained.
“The purpose of the standard is to serve as a precise typographical map for specimen labels,” he said. “The standard specifies the horizontal and vertical rule locations, font types, and font sizes on all specimen labels for certain essential, human-readable elements. These elements include the patient’s name, a unique secondary identifier, the patient’s date of birth, the specimen collection date and time, and the specimen collector’s identification,” Hawker added.
At a time when hospital lab budgets are shrinking and payers are slashing clinical laboratory test prices, implementing the new specimen label standard published by CLSI represents one way to reduce expensive lab errors, while at the same time cutting costs associated with mislabeled specimens. The upfront investment is minimal, and this is a management initiative that definitely contributes to improved patient safety in clinical laboratories and anatomic pathology groups.
Further, Charles Hawker, Ph.D., will be speaking about how lab automation and use of the CLSI bar code label standard have contributed to improved lab performance and increased patient safety at the Seventh Annual Lab Quality Confab. This annual conference is scheduled for October 1 and 2, 2013, at the Astor Crowne Plaza Hotel in New Orleans. View the agenda and register (or copy and past this URL in your browser: www.labqualityconfab.com).
—By Joseph Burns
Related Information:
Bar Codes May Have Poorer Error Rates Than Commonly Believed
Patient Misidentifications Caused By Errors in Standard Barcode Technology
What Constitutes a Correctly Labeled Specimen?
CLSI Publishes New Standard on Human-Readable Elements of Specimen Labels




I am the Phlebotomists/Lab Lead for one of the biggest hospitals in Nebraska. However I work in their Multi-Specialty Clinical Lab. 2 years ago they took away the Sunquest label printer and put in a regular printer that just prints out the patients information. I’m constantly having to double check my work to make sure I am drawing the correct color tube and having to write in the name of the test when it’s a special procedure lab order, especially when I’m pouring off the serum or plasma into an ARUP tube. This had slowed me down but my manager doesn’t think this is a problem. I have caught myself writing the wrong test on the wrong patient . I have had to implement my own generic system to track down specimens which means Log sheets for everything that comes in and out of the lab. There was an occasion where 10 patients specimens where missing and we had to back track and luckily for me that I write down everything we were able to figure out the time the courier took the specimens and 1 week later they found them in another labs cooler. There is no tracking system in my lab and I’m getting frustrated. It’s a multi specialty clinic so My brain is working double so I can make sure I’m collecting the right amount of tubes for a particular test and the right color of tube . I’m at my wits end.
Have you seen any CLSI/CAP requirements for the de-intentification of samples with barcode labels before disposal? Are labels required to be thermal? I have seen several comments before about removing labels or placing a blank label over the PI but think that sites determined that this should not be done.
Is the standard deadline a CLIA requirement or a CLSI requirement. What happens if the deadline isn’t met by laboratories.
Who created the deadline and who is going to enforce it?