Pathology groups and clinical laboratories are among the beneficiaries if the Accelerating Medicines Partnership achieves its goals
Power players in healthcare are about to invest nearly a quarter of a billion dollars to accelerate the time it takes for new medical discoveries to gain regulatory approval and enter clinical use. The emphasis will be on both therapeutic drugs and diagnostics, making this an important development for in vitro diagnostics companies and medical laboratories.
Anchors to this new initiative are the National Institutes of Health (NIH) and the Food and Drug Administration (FDA). Their partners are 10 pharmaceutical companies and six nonprofit groups. The goal is to jumpstart research to find targets for new drugs and diagnostics, noted a Genomeweb.com article.
Accelerating Medicines Partnership Is New Development Model
It is called the Accelerating Medicines Partnership (AMP) and is a new model for developing drugs with an eye to FDA approval. Nearly $230 million will be invested over the next five years to fund projects aimed at: 1) finding disease targets most likely to respond to new therapies, and 2) identifying and validating reliable disease biomarkers, according to a NIH press release.
Of particular interest to pathologists and other clinical laboratory professionals is the expectation for creation of new diagnostic tests, along with identification of reliable disease biomarkers and drug development based on translational medicine. Translational medicine is a rapidly growing discipline, particularly in biomedical research. It uses a multidisciplinary, highly collaborative, “bench-to-bedside” approach and may expedite discovery of novel diagnostics and treatments.
Advances in Several Technologies Can Speed Development of Novel Drugs
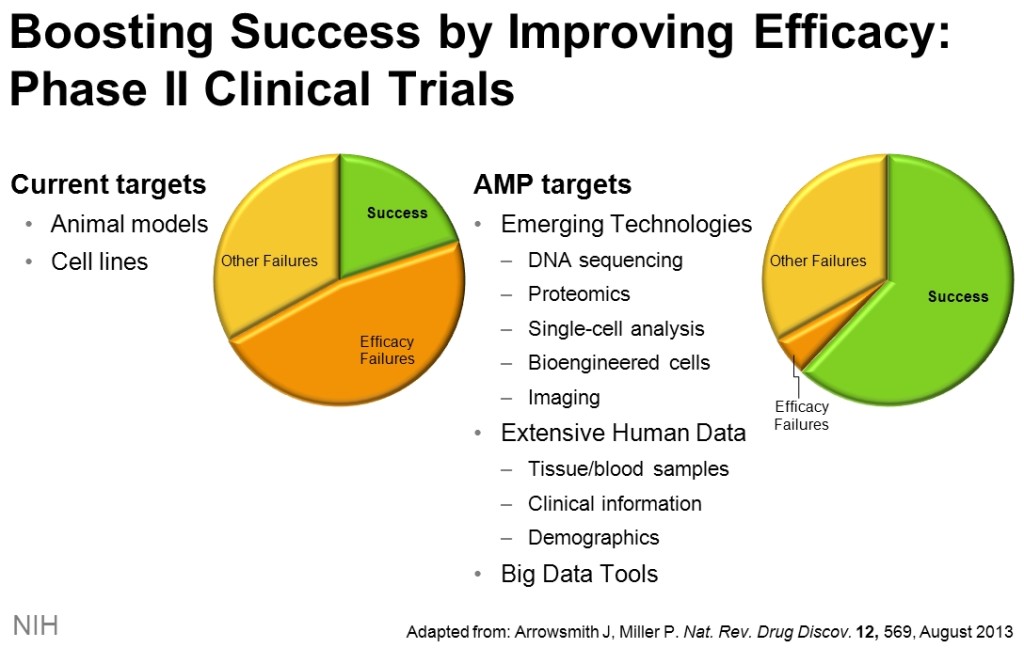
Advances in genomics, proteomics, imaging, and other technologies have resulted in identification of biological changes in genes, proteins, and other molecules that predispose individuals to disease and influence disease progression. While such biological changes hold promise for development of novel drugs, only a small number have been pursued, noted NIH Director Francis Collins, M.D., in discussing this public-private collaboration to overcome the difficult and risky business of drug development.

Francis Collins, M.D. (pictured), Director of the National Institutes for Health, says the Accelerate Medicines Partnership will advance success of drug outcomes, speed delivery of new drugs to the patients, and improve investment in drug research. (Photo is in the public domain.)
The NIH press release noted that currently drug development is a decade-long, $1-billion process, from early discovery through FDA approval. The failure rate of new drugs is 95%. “Currently, we are investing a great deal of money and time in avenues with high failure rates, while patients and their families wait,” stated Collins.
“If a drug is aimed at the wrong target, it probably won’t work against the disease it was intended to treat. Such misplaced bets waste untold time and precious resources. All who care about therapeutics agree, we need new strategies to avoid such failures.”
Creating Successful Clinical Applications that Secure FDA Clearance
While recent advances in basic research are opening “new windows of opportunity” for therapeutics, the challenge is to translate this new information into successful clinical applications that secure FDA clearance, Collins observed. “This challenge is beyond the scope of any one of us, and it’s time to work together in new ways to increase our collective odds of success. We believe this partnership is an important first step and represents the most sweeping effort to date to tackle this vital issue.”
“The AMP rallies scientific key players of the innovation ecosystem in a more unified way to address one of the key challenges to Biopharma drug discovery and development,” added Mikael Dolsten, M.D., Ph.D., President of Worldwide Research and Development at Pfizer (NYSE: PFE), an AMP participant.
“This type of novel collaboration will leverage the strengths of both industry and the NIH to ensure we expedite translation of scientific knowledge into next-generation therapies to address the urgent needs of Alzheimer’s, Type 2 diabetes and RA/lupus patients.”
Goals for Accelerate Medicines Partnership
According to the NIH press release, the primary goals of this collaboration are to:
- Improve efficiency by accelerating drug development and FDA approval, improving the success rate and lowering costs;
- Improve the drug process with a better understanding of biological and targets and valid biomarkers to facilitate clinical trials with patients most likely to respond based on their molecular disease profiles; and
- Increase the number and effectiveness of new target therapies with a better understanding of the underlying disease pathways and biological targets, improving the success rate per $1 billion invested, and generating greater returns to make drug development a more attractive business venture.
The AMP will begin by launching pilot projects in three disease areas: Alzheimer’s disease, Type 2 diabetes, and autoimmune disorders, including rheumatoid arthritis (RA) and systemic lupus erythematosus (lupus). Scientists participating in these pilot projects will focus on characterizing biomarkers for these diseases and identifying biological targets most likely to respond to new therapies.
Partnership Includes NIH, FDA, Pharma Industry and Patient Groups
Besides the government agencies, the commercial AMP partners are:
- AbbVie (NYSE: ABBV)
- Biogen Idec (NASDAQ: BLLB)
- Bristol-Myers Squibb (NYSE: BMY)
- GlaxoSmithKline (NYSE: GSK)
- Johnson & Johnson (NYSE: JNJ)
- Eli Lilly & Co. (NYSE: LLY)
- Merck (NYSE: MRK)
- Pfizer (NYSE: PFE)
- Sanofi (NYSE: SNY)
- Takeda Pharmaceutical Company Limited
Also participating in the Alliance for Medicine Development are these non-profit organizations:
- Alzheimer’s Association
- American Diabetes Association
- Lupus Foundation of America
- Geoffrey Beene Foundation
- Rheumatology Research Foundation
- USAgainstAlzheimer’s
- PhRMA
Highly collaborative steering committees composed of representatives from the public and private partners will provide oversight for each disease area. These steering committees will be managed by the Foundation for the NIH (FNIH) under the direction of an AMP executive committee composed of leaders from the NIH, Pharma industry, FDA and patient advocacy groups.
IVD Manufacturers and Medical Laboratories Could Benefit
The implications of this $230 million-dollar development initiative could be substantial for the IVD industry and medical laboratories. Should the Alliance for Medicine Development succeed in both shortening the time to FDA clearance and increasing the success rate for new therapeutic drugs and diagnostic tests, it can be expected that clinical laboratories and anatomic pathology groups will be able to offer these new diagnostic tests in ways that improve patient outcomes, while favorably reducing the overall cost of a patient’s healthcare encounter.
—by Patricia Kirk
Related Information:
NIH, industry and non-profits join forces to speed validation of disease targets
NIH, Industry Launch $230M Translational Drugs, Diagnostics Push