Though response was limited in Dark Daily’s poll, the message from respondents was overwhelmingly negative on LDT regulation by the FDA
Most respondents to a recent industry survey said that should Food and Drug Administration (FDA) approval be required in the future for laboratory developed tests (LDTs), innovation will suffer.
This conclusion echoes what opponents of the Verifying Accurate Leading-Edge IVCT Development Act (VALID Act) have argued will happen should that proposed bill become law.
In the survey, which was conducted by Dark Daily, 91% of respondents said FDA pre-market approval of LDTs would decrease clinical laboratories’ ability to bring innovative tests to market. (See Figure 1.) The other 9% felt it would have no effect on innovation. Zero of the respondents said FDA involvement would increase innovative tests.
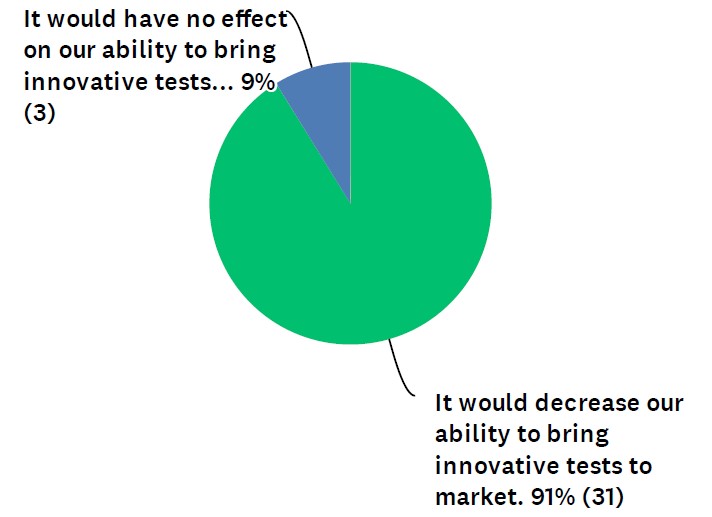
“Development of LDT tests has been the mission of most of the labs, and it meets the need for patient care,” noted one respondent in the survey. “Moving LDTs under FDA will create more obstacles for labs to offer the tests.”
To be fair, the survey had limited responses—34 in total. The poll went out to thousands of Dark Daily readers. We found that response rate surprising given how many labs will be affected if the VALID Act becomes law.
An LDT is a proprietary diagnostic test developed and performed by an individual clinical laboratory. In academic medical center laboratories, LDTs often address unmet clinical needs. The Clinical Laboratory Improvement Amendments of 1988 (CLIA) generally regulates LDTs.
VALID Act vs. VITAL Act Hinges on LDT Oversight
The VALID Act is a bipartisan bill that proposes FDA oversight of laboratory developed tests. The bill continues to make its way through the U.S. Senate and the House of Representatives.
A counterproposal called the Verified Innovative Testing in American Laboratories Act (VITAL Act) is also before Congress but has less momentum behind it. The VITAL Act seeks to keep LDTs under CLIA while also calling for reforms to account for modern lab tests.
See our past reporting in Dark Daily for a detailed comparison of the VALID Act and VITAL Act.
Looking at survey results, 80% “strongly disagreed” or “disagreed” that new LDT requirements, such as those found in the VALID Act, are needed. (See Figure 2.)
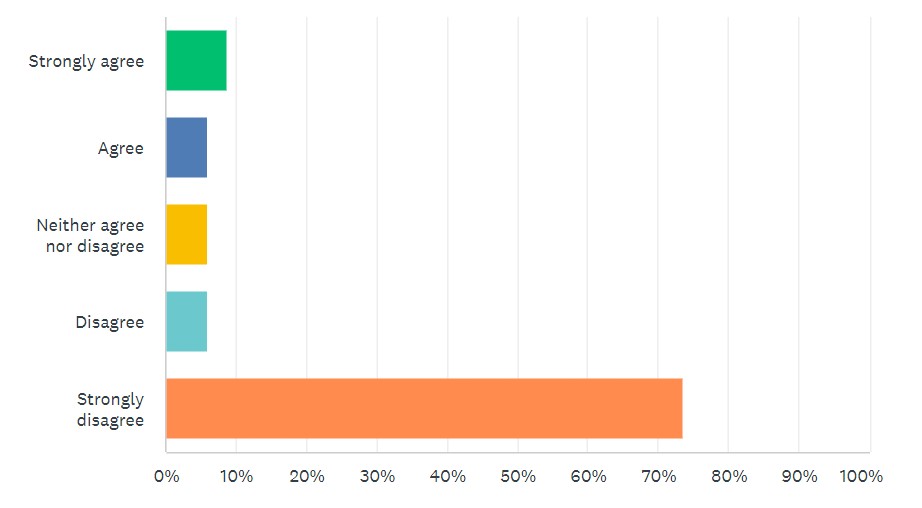
By comparison, 65% “strongly agreed” or “agreed” with modernizing CLIA requirements for LDTs, as called for in the VITAL Act.
Those numbers shifted somewhat depending on the lab setting of the respondent. For example, just looking at commercial labs, opposition to the VALID Act remained similar, but support for modernizing CLIA jumped up to 88%. When looking at just hospitals, independent labs, and academic labs, numbers for both topics remained consistent.
When filtering the answers, the number of lab employees in a setting had little effect on survey results.
Political Battle Continues Over Laboratory Developed Tests
Clinical laboratory industry groups and others have been amassing to oppose or support the VALID Act. For example, the Advanced Medical Technology Association and The Pew Charitable Trusts are behind the bill.
However, the American Association for Clinical Chemistry, Association for Molecular Pathology, and new Coalition for Innovative Laboratory Testing are against the VALID Act.
Congress may attach the VALID Act to the authorization vote for the Medical Device User Fee Agreement V (MDUFA). At this point, discussions on MDUFA remain in congressional committees.
—Scott Wallask
Related Information:
What is a laboratory developed test?
LDT Regulation: New Survey Asks Readers for Their Views About Two Bills Before Congress
MDUFA V Agreement Enables Bold MedTech Vision to Become Reality



