Nov 14, 2018 | Coding, Billing, and Collections, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
Physicians in Saskatchewan called for changes after wait times for anatomic pathology test results reached six weeks or more
Anatomic pathologist and histopathologist shortages have plagued the single-payer healthcare systems in Canada and the United Kingdom (UK) in recent years. The consequence is increased wait times for physicians in both countries to receive medical laboratory test results, which increases wait times across the entire healthcare continuum.
However, one Canadian province significantly reduced a backlog that had pushed wait times for surgical pathology test results to six weeks or more. It did this by having its pathologists perform first-stage examinations normally completed by pathology assistants or medical technologists.
The Saskatchewan Health Authority (SHA) announced in October it had cleared nearly half of the 2,600-plus biopsies that were waiting to be processed at hospital labs in Regina and Saskatoon, the Regina Leader-Post reported.
“I think we’ve been making amazing progress in the work,” Lenore Howey, Executive Director of Laboratory Services at SHA, told the newspaper. “It’s always good to take time to know and understand your process, so that we can put the right resources in the right places.”
Getting Anatomic Pathologists Involved
Howey stated the SHA cleared cases by having pathologists “assist with the work in the first phase”—or gross examination stage—of a biopsy. This is the part of the process during which pathology assistants or medical laboratory technologists typically record the size, weight, and description of a specimen and look for pathological changes.
In addition, the SHA hired an additional pathologist assistant and three histology/cytology technologists—one on a permanent basis and two on a temporary basis. Other improvements include:
- Working toward resolving problems with voice recognition transcription software being piloted in Regina for the gross examination phase of processing; and;
- Implementing an electronic specimen tracking system in Saskatoon, which eventually also may be used in Regina.
Physicians Express Dissatisfaction with Wait Times
Physicians attending the Saskatchewan Medical Association’s Spring Representative Assembly in May raised the backlog issue with Health Minister Jim Reiter, complaining about the impact on patient care. At that point, the backlog of pathology cases had hit 1,662 in Regina, while Saskatoon’s caseload totaled 1,005. Many of these biopsies involve cancer patients, thus delaying a diagnosis and the start of an appropriate treatment for these patients.
“I’m trying to get things done as expeditiously as possible,” urologist Francisco Garcia, MD, told the Leader-Post, “but for the first five or six weeks, I’m handcuffed in terms of what I’m able to do.”
Now, thanks to SHA’s efforts, as of Oct. 2 specimens in progress dropped to 785 in Regina and 748 in Saskatoon. Both numbers are within range of SHA’s target of 750.

“We do not have a backlog right now,” Lenore Howey, Executive Director of Laboratory Services at SHA, told the Leader-Post. “Our system is very stable, but we do have checks and balances to put in place so that we would never get there again, which we didn’t have prior.” (Photo copyright: Saskatchewan Health Authority.)
Wait Times Impacting Patient Care Worldwide
While Saskatchewan appears to have solved its most recent pathology reporting issue, this is not the first time the province has dealt with delays in lab testing reports. In 2011, Dark Daily reported on lengthy turnaround times for anatomic pathology test reports that averaged more than 12 days, which was blamed on shortage of pathologists dating back to 2001. (See, “Pathologist Shortage and Delays in Lab Test Reports Get Publicity in Saskatchewan,” August 15, 2011.)
And in October, Dark Daily reported that cancer patients in the UK are experiencing record waiting times for treatments, with more than 3,000 people waiting longer than two months to begin care, iNews reported. Delays there are being blamed in part on severe shortages of pathology staff. A 2017 workforce survey by the Royal College of Pathologists reported that only 3% of the National Health Service (NHS) histopathology departments responding to the survey had adequate staff. (See, “Shortage of Histopathologists in the United Kingdom Now Contributing to Record-Long Cancer-Treatment Waiting Times in England,” October 31, 2018.)
“Making sure pathology services can cope with current and future demand is essential if we are to ensure early diagnosis and improve outcomes for patients,” Jo Martin, PhD, President of the Royal College of Pathologists, told the BBC.
Increased workloads due to new NHS screening programs and an approaching retirement crisis—a quarter of all histopathologists in the UK are aged 55 or over—has caused the Royal College of Pathologists to call for more funded training places, better IT systems, and further investment in pathology services.
While the US healthcare system is not currently experiencing a shortage of clinical laboratory staff or anatomic pathologists, shortages in other countries illustrate the impact any delay in reporting results can have on patient care.
—Andrea Downing Peck
Related Information:
Backlog of Pathology Tests Cleared in Province
Technology and Staff Shortages Contribute to Biopsy Backlog
Pathology Staff Shortages Causing Delays to Cancer Diagnosis, Says Report
Cancer Waiting Times at their Worst Ever Level
Histopathology Workforce Survey 2018
Pathologists Shortage ‘Delaying Cancer Diagnosis’
Pathologists Shortage and Delays in Lab Test Reports Get Publicity in Saskatchewan
Shortage of Histopathologists in the United Kingdom Now Contributing to Record-Long Cancer-Treatment Waiting Times in England
Nov 2, 2018 | Coding, Billing, and Collections, Compliance, Legal, and Malpractice, Digital Pathology, Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
Patient privacy, ethics of monetizing not-for-profit data, and questions surrounding industry conflicts appear after the public announcement of an arrangement to grant exclusive access to academic pathology slides and samples
Clinical laboratories and anatomic pathology groups already serve as gatekeepers for a range of medical data used in patient treatments. Glass slides, paraffin-embedded tissue specimens, pathology reports, and autopsy records hold immense value to researchers. The challenge has been how pathologists (and others) in a not-for-profit academic center could set themselves up to potentially profit from their exclusive access to this archived pathology material.
Now, a recent partnership between Memorial Sloan Kettering Cancer Center (MSK) and Paige.AI (a developer of artificial intelligence for pathology) shows how academic pathology laboratories might accomplish this goal and serve a similar gatekeeper role in research and development using the decades of cases in their archives.
The arrangement, however, is not without controversy.
New York Times, ProPublica Report
Following an investigative report from the New York Times (NYT) and ProPublica, pathologists and board members at MSK are under fire from doctors and scientists there who have concerns surrounding ethics, exclusivity, and profiting from data generated by physicians and but owned by MSK.
“Hospital pathologists have strongly objected to the Paige.AI deal, saying it is unfair that the founders received equity stakes in a company that relies on the pathologists’ expertise and work amassed over 60 years. They also questioned the use of patients’ data—even if it is anonymous—without their knowledge in a profit-driven venture,” the NYT article states.
Prominent members of MSK are facing scrutiny from the media and peers—with some relinquishing stakes in Paige.AI—as part of the backlash of the report. This is an example of the perils and PR concerns lab stakeholders might face concerning the safety of data sharing and profits made by medical laboratories and other diagnostics providers using patient data.
Controversy Surrounds Formation of Paige.AI/MSK Partnership
In February 2018, Paige.AI announced closing the deal on a $25-million round of Series A funding, and in gaining exclusive access to 25-million pathology slides and computational pathology intellectual property held by the Department of Pathology at Memorial Sloan Kettering. Coverage by TechCrunch noted that while MSK received an equity stake as part of the licensing agreement, they were not a cash investor.
Creation of the company involved three hospital insiders and three additional board members with the hospital itself established as part owner, according to STAT.
Unnamed officials told the NYT that board members at MSK only invested in Paige.AI after earlier efforts to generate outside interest and investors were unsuccessful. NYT’s coverage also notes experts in non-profit law and corporate governance have raised questions as to compliance with federal and state laws that govern nonprofits in light of the Paige.AI deal.
Growing Privacy Fallout and Potential Pitfalls for Medical Labs
The original September 2018 NYT coverage noted that Klimstra intends to divest his ownership stake in Paige.AI. Later coverage by NYT in October, notes that Democrat Representative Debbie Dingell of Michigan submitted a letter questioning details about patient privacy related to Paige.AI’s access to MSK’s academic pathology resources.
Privacy continues to be a focus for both media and regulatory scrutiny as patient data continues to fill electronic health record (EHR) systems as well as research and commercial databases. Dark Daily recently covered how University of Melbourne researchers demonstrated how easily malicious parties might reidentify deidentified data. (See “Researchers Easily Reidentify Deidentified Patient Records with 95% Accuracy; Privacy Protection of Patient Test Records a Concern for Clinical Laboratories”, October 10, 2018.)
According to the NYT, MSK also issued a memo to employees announcing new restrictions on interactions with for-profit companies with a moratorium on board members investing in or holding board positions in startups created within MSK. The nonprofit further noted it is considering barring hospital executives from receiving compensation for their work on outside boards.
However, MSK told the NYT this only applies to new deals and will not affect the exclusive deal between Paige.AI and MSK.
“We have determined,” MSK wrote, “that when profits emerge through the monetization of our research, financial payments to MSK-designated board members should be used for the benefit of the institution.”
There are no current official legal filings regarding actions against the partnership. Despite this, the arrangement—and the subsequent fallout after the public announcement of the arrangement—serve as an example of pitfalls medical laboratories and other medical service centers considering similar arrangements might face in terms of public relations and employee scrutiny.
Risk versus Reward of Monetizing Pathology Data
While the Paige.AI situation is only one of multiple concerns now facing healthcare teams and board members at MSK, the events are an example of risks pathologists take when playing a role in a commercial enterprise outside their own operations or departments.
In doing so, the pathologists investing in and shaping the deal with Paige.AI brought criticism from reputable sources and negative exposure in major media outlets for their enterprise, themselves, and MSK as a whole. The lesson from this episode is that pathologists should tread carefully when entertaining offers to access the patient materials and data archived by their respective anatomic pathology and clinical laboratory organizations.
—Jon Stone
Related Information:
Sloan Kettering’s Cozy Deal with Start-Up Ignites a New Uproar
Paige.AI Nabs $25M, Inks IP Deal with Sloan Kettering to Bring Machine Learning to Cancer Pathology
Sloan Kettering Executive Turns Over Windfall Stake in Biotech Start-Up
Cancer Center’s Board Chairman Faults Top Doctor over ‘Crossed Lines’
Memorial Sloan Kettering, You’ve Betrayed My Trust
LVHN Patient Data Not Shared with For-Profit Company in Sloan Kettering Trials
Researchers Easily Reidentify Deidentified Patient Records with 95% Accuracy; Privacy Protection of Patient Test Records a Concern for Clinical Laboratories
Oct 10, 2018 | Coding, Billing, and Collections, Compliance, Legal, and Malpractice, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
Protecting patient privacy is of critical importance, and yet researchers reidentified data using only a few additional data points, casting doubt on the effectiveness of existing federally required data security methods and sharing protocols
Clinical laboratories and anatomic pathologists know the data generated by their diagnostics and testing services constitute most of a patient’s personal health record (PHR). They also know federal law requires them to secure their patients’ protected health information (PHI) and any threat to the security of that data endangers medical laboratories and healthcare practices as well.
Therefore, recent coverage in The Guardian which reported on how easily so-called “deidentified data” can be reidentified with just a few additional data points should be of particular interest to clinical laboratory and health network managers and stakeholders.
Risky Balance Between Data Sharing and Privacy
In December 2017, University of Melbourne (UM) researchers, Chris Culnane, PhD, Benjamin Rubinstein, and Vanessa Teague, PhD, published a report with the Cornell University Library detailing how they reidentified data listed in an open dataset of Australian medical billing records.
“We found that patients can be re-identified, without decryption, through a process of linking the unencrypted parts of the record with known information about the individual such as medical procedures and year of birth,” Culnane stated in a UM news release. “This shows the surprising ease with which de-identification can fail, highlighting the risky balance between data sharing and privacy.”
In a similar study published in Scientific Reports, Yves-Alexandre de Montjoye, PhD, a computation private researcher, used location data on 1.5 million people from a mobile phone dataset collected over 15 months to identify 95% of the people in an anonymized dataset using four unique data points. With just two unique data points, he could identify 50% of the people in the dataset.
“Location data is a fingerprint. It’s a piece of information that’s likely to exist across a broad range of data sets and could potentially be used as a global identifier,” Montjoye told The Guardian.
The problem is exacerbated by the fact that everything we do online these days generates data—much of it open to the public. “If you want to be a functioning member of society, you have no ability to restrict the amount of data that’s being vacuumed out of you to a meaningful level,” Chris Vickery, a security researcher and Director of Cyber Risk Research at UpGuard, told The Guardian.
This privacy vulnerability isn’t restricted to just users of the Internet and social media. In 2013, Latanya Sweeney, PhD, Professor and Director at Harvard’s Data Privacy Lab, performed similar analysis on approximately 579 participants in the Personal Genome Project who provided their zip code, date of birth, and gender to be included in the dataset. Of those analyzed, she named 42% of the individuals. Personal Genome Project later confirmed 97% of her submitted names according to Forbes.

In testimony before the Privacy and Integrity Advisory Committee of the Department of Homeland Security (DHS), Latanya Sweeney, PhD (above), Professor and Director at Harvard’s Data Privacy Lab stated, “One problem is that people don’t understand what makes data unique or identifiable. For example, in 1997 I was able to show how medical information that had all explicit identifiers, such as name, address and Social Security number removed could be reidentified using publicly available population registers (e.g., a voter list). In this particular example, I was able to show how the medical record of William Weld, the Governor of Massachusetts of the time, could be reidentified using only his date of birth, gender, and ZIP. In fact, 87% of the population of the United States is uniquely identified by date of birth (e.g., month, day, and year), gender, and their 5-digit ZIP codes. The point is that data that may look anonymous is not necessarily anonymous. Scientific assessment is needed.” (Photo copyright: US Department of Health and Human Services.)
These studies reveal that—regardless of attempts to create security standards—such as the Privacy Rule in the Health Insurance Portability and Accountability Act of 1996 (HIPAA)—the sheer amount of available data on the Internet makes it relatively easy to reidentify data that has been deidentified.
The Future of Privacy in Big Data
“Open publication of deidentified records like health, census, tax or Centrelink data is bound to fail, as it is trying to achieve two inconsistent aims: the protection of individual privacy and publication of detailed individual records,” Dr. Teague noted in the UM news release. “We need a much more controlled release in a secure research environment, as well as the ability to provide patients greater control and visibility over their data.”
While studies are mounting to show how vulnerable deidentified information might be, there’s little in the way of movement to fix the issue. Nevertheless, clinical laboratories should consider carefully any decision to sell anonymized (AKA, blinded) patient data for data mining purposes. The data may still contain enough identifying information to be used inappropriately. (See Dark Daily, “Coverage of Alexion Investigation Highlights the Risk to Clinical Laboratories That Sell Blinded Medical Data,” June 21, 2017.)
Should regulators and governments address the issue, clinical laboratories and healthcare providers could find more stringent regulations on the sharing of data—both identified and deidentified—and increased liability and responsibility regarding its governance and safekeeping.
Until then, any healthcare professional or researcher should consider the implications of deidentification—both to patients and businesses—should people use the data shared in unexpected and potentially malicious ways.
—Jon Stone
Related Information:
‘Data Is a Fingerprint’: Why You Aren’t as Anonymous as You Think Online
Research Reveals De-Identified Patient Data Can Be Re-Identified
Health Data in an Open World
The Simple Process of Re-Identifying Patients in Public Health Records
Harvard Professor Re-Identifies Anonymous Volunteers in DNA Study
How Someone Can Re-Identify Your Medical Records
Trading in Medical Data: Is this a Headache or An Opportunity for Pathologists and Clinical Laboratories
Coverage of Alexion Investigation Highlights the Risk to Clinical Laboratories That Sell Blinded Medical Data
Apr 20, 2018 | Coding, Billing, and Collections, Compliance, Legal, and Malpractice, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing
Though ACA reforms may have slowed healthcare spending, rapidly increasing deductibles and cost sharing requirements have many experts questioning if patients can afford care at all, despite the increased availability of insurance coverage
Much of the debate surrounding efforts to replace and repeal the Affordable Care Act (ACA) has centered on premiums as a central facet of out-of-pocket spending. However, new data from a Kaiser Family Foundation (KFF) survey reveals that premiums are only one factor affecting consumers’ ability to pay healthcare bills. High-deductible health plans (HDHPs) are another culprit. This directly impacts clinical laboratories and anatomic pathology groups that find revenues down as more American’s avoid costs by delaying or opting out of testing and treatments.
The KFF report highlights both the complexity of managing healthcare costs and how the current focus on premium prices might miss other important considerations that make healthcare inaccessible to many Americans.
High Deductibles and Consumers’ Lack of Savings
An increasing number of insurance plans now include high deductibles—particularly in the individual markets, though employer-based insurance plans are experiencing steady increases as well.
This leaves consumers facing larger bills and making tough decisions about whether their healthcare is affordable—even with insurance.
When healthcare consumers cannot afford the out-of-pocket costs of healthcare, they are less likely to schedule wellness visits, adhere to treatments, or follow through on physician-ordered clinical laboratory tests they don’t consider essential to their well-being or simply cannot afford.
Even when they follow protocols and recommendations, that does not mean patients will be able to pay medical laboratories for tests performed, or anatomic pathology groups for specialized services, when the bill comes due.
The Ever-Growing Deductible Dilemma
In its 2017 study, “Do Health Plan Enrollees have Enough Money to Pay Cost Sharing?,” the KFF compares median data on liquid assets from 6,254 single and multi-person households—spanning a range of incomes and age brackets—to the average cost of both standard employer-based insurance and individual market insurance deductibles.
They further note that their data modeling and estimates present a “conservative estimate,” because chronic conditions might cause an extended period of out-of-pocket spending, and that median assets might not be available at a single time or throughout the year.
Concerning a previous 2016 KFF study on high-deductible insurance plans, the authors noted in a press release, “In 2016, 83% of covered workers face a deductible for single coverage, which averages $1,478. That’s up $159 or 12% from 2015, and $486 or 49% since 2011. The average deductible for workers who face one is higher for workers in small firms (three to 199 employers) than in large firms ($2,069 vs. $1,238).”

In the press release following KFF’s 2016 survey, Drew Altman, CEO (above), Kaiser Family Foundation, noted, “We’re seeing premiums rising at historically slow rates, which helps workers and employers alike, but it’s made possible in part by the more rapid rise in the deductibles workers must pay.” (Image copyright: Kaiser Family Foundation.)
In their latest look at deductibles and out-of-pocket spending, the KFF study authors note, “About half (53%) of single-person non-elderly households could pay the $2,000 from their liquid assets towards cost sharing, and only 37% could pay $6,000, which … was less than the maximum out-of-pocket limit for single coverage in 2016. For multi-person families, 47% could pay $4,000 from their liquid assets for cost sharing, while only 35% could pay $12,000.”
This sets the stage for the grim picture now facing many Americans. Despite increased access to medical insurance, being able to use the insurance to obtain care can be a struggle for a sizeable part of the lower to middle class population.
Creating a More Affordable Future for Healthcare
Data from the Q1 National Health Interview Survey (NHIS) conducted by the Centers for Disease Control and Prevention (CDC) show that growth in high-deductible plans might skew these numbers further still. They found that the number of persons under the age of 65 enrolled in HDHPs increased from 25.3% in 2010 to 40.0% in the first quarter of 2016 despite uninsured rates dropping from 22.3% to 11.9% over the same period.
In the 2017 study, KFF outlines the complexity of the issue: “There are significant differences across the income spectrum … For example, 63% of multi-person households with incomes of 400% of poverty or more could pay $12,000 from liquid assets for cost sharing, compared with only 18% of households with incomes between 150% and 400% of poverty, and 4% of households with incomes below 150% of poverty.”
While there are no simple answers to address today’s increasing deductibles, KFF emphasizes the importance of looking at the bigger picture.
“Much of the discussion around affordability has centered on premium costs. A broader notion of affordability will have to focus on the ability of families,” they note. “To adequately address the issue of affordability of health insurance, reform proposals should be evaluated on the affordability of out-of-pocket costs, especially for low and moderate-income families, and be sensitive to the financial impacts that high cost sharing will have on financial wellbeing.”
In the meantime, lack of access to preventative care and regular checkups can increase long-term healthcare costs and health risks, creating a spiral of financial concerns for patients as well as the healthcare professionals and the clinical laboratories serving them.
—Jon Stone
Related Information:
The Biggest Health Issue We Aren’t Debating
Do Health Plan Enrollees Have Enough Money to Pay Cost Sharing?
Average Annual Workplace Family Health Premiums Rise Modest 3% to $18,142 in 2016; More Workers Enroll in High-Deductible Plans with Savings Option Over Past Two Years
Americans Are Facing Rising Out-of-Pocket Healthcare Costs—Here’s Why
Americans’ Out-of-Pocket Healthcare Costs Are Skyrocketing
Americans Are Shouldering More and More of Their Healthcare Costs
Medicare Out-of-Pocket Costs Seen Rising to Half of Senior Income
Consumer Reaction to High-Deductible Health Plans and Rising Out-of-Pocket Costs Continues to Impact Physicians and Clinical Laboratories
Because of Sizeable Deductibles, More Patients Owe More Money to Clinical Pathology Laboratories, Spurring Labs to Get Smarter about Collecting from Patients
Growth in High Deductible Health Plans Cause Savvy Clinical Labs and Pathology Groups to Collect Full Payment at Time of Service
Jan 24, 2018 | Coding, Billing, and Collections, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
Because many Americans are ‘concerned’ about how they would pay an unexpected medical bill, they now are seeking upfront information about treatment costs and financing options
Clinical laboratories and anatomic pathology groups that depend on reliable sources of patient and test referrals are being impacted by a reduction in patients seeking care due to rising costs. Evidence continues to mount that high deductible health plans (HDHPs) and the overall rising cost of healthcare are straining Americans’ finances. This is causing them to delay payments, question treatment costs, and investigate payment options.
These trends underscore the need for clinical laboratories and pathology groups to have point-of-service collection strategies in place or risk declining revenues.
Study Highlights Increasing Consumer Healthcare Costs
JPMorgan Chase Institute’s Healthcare Out-of-pocket Spending Panel (JPMCI HOSP) recently studied the healthcare cost burden on 2.3-million de-identified Chase checking account holders aged 18 to 64. In a report titled, “Paying Out-of-Pocket: The Healthcare Spending of 2 Million US Families,” Chase noted the following key indicators that predict continued decline in healthcare revenues as patients’ costs increase:
· “A clear correlation exists between timing of healthcare payments and an account-holder’s ability to pay, with the largest payments taking place in the years and months with increased liquid assets. This finding emphasizes the clear link between a family’s financial health and their access to healthcare services. The report found a clear spike in payments during the months of March and April, when nearly 80% of tax filers receive tax refunds.
· “There is significant variation of out-of-pocket expenses among and within states, emphasizing the important role of states in shaping healthcare policy. Colorado families spent the most out of pocket, while families in Louisiana spent the most as a percent of income. California was among the lowest in terms of both raw dollar amounts and payments as a share of income. As part of this report, the JPMorgan Chase Institute has created online data visualization assets to illustrate these disparities and is providing downloadable payment data with information broken down to metro and county levels.
· “Out-of-pocket payments grew each year since 2013, but have remained a stable share of income, also known as “burden.” However, women, low-income families and pre-seniors are bearing the highest cost burden. The finding merits further study to establish whether these higher payments represent broader healthcare utilization or a clear expense burden for populations that can afford it the least.
· “Families that are in the top 10% of healthcare spend in a given year tend to remain the highest spenders on a year-over-year basis, emphasizing the substantial cost of chronic conditions and long-term healthcare needs.
· “Doctor, dental, and hospital payments accounted for more than half of out-of-pocket payments. While doctor payments accounted for the greatest volume of expenditures, dental and hospital payments were much more significant in terms of expense.”
In a news release, Diana Farrell, President and CEO, JPMorgan Chase Institute points out that, “The reality is that many American families don’t have the cash buffer to withstand the volatility created by out-of-pocket healthcare payments, and we need to better understand the correlation between financial health and physical health.”
The JPMorgan Chase Institute report, released September 19, 2017, came on the heels of the Kaiser Family Foundation (KFF)/Health Research and Education Trust (HRET) 2017 Employer Health Benefits Survey. This annual benchmark survey indicates workers on average now contribute $5,714 annually toward their family premiums, which average $18,764, and that employees at firms with fewer than 200 workers contribute as much as $6,814 on average.
The findings of this survey will be useful for those clinical laboratories and anatomic pathology groups developing business and clinical strategies to serve the growing numbers of patients who are covered by high-deductible health plans. The KFF/HRET survey highlighted the impact growth of HDHPs is having on workers:
· 81% of covered workers were in plans with an annual deductible, up from 59% in 2007 and 72% in 2012;
· The average deductible amounts for workers with employer-based coverage also is increasing steadily, rising from $616 in 2007 to $1,505 in 2017.

A JPMorgan Chase Institute study of family healthcare spending (not including premium payments) shows out-of-pocket costs varied widely in the US in 2016, both across and within states. Average spending ranged from a low of $596/year (California) to a high of $916/year (Colorado) in the 23 states where there are Chase retail banking branches. (Photo copyright: JPMorgan Chase Institute/Business Insider.)
Jay Bhatt, DO, President of KFF/HRET, and Senior Vice President and Chief Medical Officer at the American Hospital Association, notes that while some cost increases appear to be slowing, policymakers should continue seeking ways to reduce the burden on healthcare consumers.
“This year’s findings continue a positive run of a slowing in premium increases and in the growth of healthcare costs overall,” Bhatt states in a KFF news release. “As policymakers and providers continue to work to improve healthcare, ensuring it remains affordable and accessible is critically important.”
Importance of Providing Pricing and Payment Options
These results help explain why 42% of respondents to HealthFirst Financial’s Patient Survey stated they are “very concerned” or “concerned” about whether they could pay out-of-pocket medical expenses during the next two years. For example:
· More than half (53%) of those surveyed were concerned about how to pay a medical bill of less than $1,000;
· 35% indicated they would find paying a bill less than $500 difficult; and,
· 16% were worried about paying a bill less than $250.
Such financial worries will likely impact revenues at clinical laboratories as well as medical doctor’s offices. They also explain why 77% of healthcare consumers who participated in the HealthFirst Financial survey responded that it’s “important” or “very important” to know their costs before treatment.
Additionally, 53% of those surveyed want to discuss financing options before receiving care. However, according to the survey, just 18% of providers discussed payment options.
The study also found 40% of millennials would likely switch to a different provider offering low- or zero-interest financing for medical bills.
“These findings highlight a huge gap in what patients want and what hospitals, medical groups, and other healthcare providers are delivering,” KaLynn Gates, JD, President and Corporate Counsel of HealthFirst Financial, said in a news release. “Providers that care for the financial as well as clinical needs of their communities are much more likely to thrive in this era of rising out-of-pocket costs and growing competition for patients among traditional and non-traditional providers.”
Clinical Laboratories and Anatomic Pathology Groups Are Particularly Challenged
In “Hospitals, Pathology Groups, Clinical Labs Struggling to Collect Payments from Patients with High-Deductible Health Plans,” Dark Daily reported that clinical laboratories and pathology groups face particular challenges because, as patients become responsible for more of their healthcare bills, many labs are not prepared for collecting full payments from patients on HDHPs. Nor are they prepared for reduction in test ordering, as patients opt to not follow through with prescribed tests and treatments to save money.
These recent reports are another strong indicator of how critical it is for medical laboratories and pathology groups to develop tools and workflow processes for collecting payments upfront from patients with HDHPs.
—Andrea Downing Peck
Related Information:
Out-of-Pocket Healthcare Costs Straining Americans’ Finances
Paying Out-of-Pocket: The Healthcare Spending of 2 Million US Families
Here’s How Much People Spend on Healthcare by State
2017 Employer Health Benefits Survey
Premiums for Employer-Sponsored Family Health Coverage Rise Slowly for Sixth Straight Year, up 3%, but Averaging $18,764 in 2017
HealthFirst Financial Patient Survey: It’s Never Too Soon to Communicate Pricing and Payment Options
From Millennials to Boomers, Patients Want to Discuss Healthcare Pricing and Payment Options before Treatment
Hospitals, Pathology Groups, Clinical Labs Struggle to Collect Payments from Patients with High-Deductible Health Plans
Jan 17, 2018 | Coding, Billing, and Collections, Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Pathology, Laboratory Testing, Management & Operations
Moving to market are the newest generation of LIMS products designed to serve clinical laboratories while supporting quality reporting initiatives and new sources of revenue
It was Bob Dylan who made a big hit out of the song, “The Times, They Are A-Changin’.” The same could be said for the next generation of software products designed for use by medical laboratories.
To be fully successful, these next-generation laboratory information management systems (LIMS) must be radically different than the generations that came before. For example, medical laboratories are frustrated with the many limitations of older LIS products that still incorporate software technologies that date back to the 1980s and 1990s, such as MUMPS, which stands for Massachusetts General Hospital Utility Multi-Programming System.
But the newest LIMS products must do more than simply incorporate the latest technologies in software and cloud-based services. They must support all the ways that clinical laboratories and anatomic pathology groups generate increased revenue. More specifically, all medical laboratories will be generating vast quantities of molecular and genetic data. Therefore, an effective LIMS must be capable of capturing that data while also enabling the lab to perform certain healthcare big data analyses in support of the referring physicians and parent hospitals.
There also will be the need for medical laboratories to use their LIMS capabilities to support the data reporting requirements of Medicare and private health insurers. Payers increasingly want providers to report their quality monitoring, patient outcomes, and certain cost-of-care parameters. All these are functions that older LIS (laboratory information systems) products were not developed to provide.
Anatomic pathology group stakeholders and clinical laboratory managers understand the vital importance of their LIMS. Laboratory and healthcare workflows depend on the system’s:
- efficiency;
- scalability that supports the growth of the lab and medical practice; and,
- flexibility to interface with modern, point-of-care telehealth technologies in ways that enable labs and practices to engage in today’s precision medicine healthcare initiatives.
The more immediate need is for a LIMS to be capable of supporting Medicare’s Quality Payment Programs (QPPs), primarily the MACRA Merit-based Incentive Payments System (MIPS). Most physicians, including pathologists, will participate in MIPS. The first Medicare incentives or penalties will be paid next year, based on 2018 metrics and performance.
Given all these changing demands of advanced software technologies and the need for medical laboratories to participate in various value-based revenue programs, how might a LIMS empower labs to ensure success and increased revenue?
Quality Payment Programs and Merit-based Incentives
As part of the shift toward value-based care, the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) works to drive down costs and increase quality within both care and laboratory environments. MACRA establishes a data-driven payment system to reimburse service providers based on the outcome of services and care episodes, instead of the volume of services delivered or billed.
Combined with reduced payments, MACRA’s incentives and penalties, and Medicare’s QPP/MIPS payment programs, pressure has been increased on healthcare providers and medical laboratories alike. Thus, technology that gives labs a competitive edge is essential for thriving in an ever-evolving and increasingly competitive marketplace.
Meeting MACRA Goals with a Laboratory Information Management System
While electronic health record (EHR) systems have helped to consolidate patient protected health information (PHI), they do little to address the real-time creation of laboratory data and the accessibility of the massive volume of lab-related data stored in the average patient’s medical files.
A LIMS, however, helps to consolidate all this data in an easily accessible and powerful system. Some LIMS even combine with telehealth technologies to make data actionable and available at the point-of-care.
In this type of LIMS, laboratories, physicians, and other care providers all access the same dataset to ensure information is relayed quickly and efficiently. Interaction takes place using cloud-based interfaces, such as mobile apps or web portals. This ensures access to patient data and laboratory test results in a variety of locations without dependence on proprietary communications systems or hardware.
From bustling ERs and surgical wards to phlebotomists visiting long-term care facilities and mobile clinics, collecting and retrieving data becomes streamlined and accessible virtually anywhere.
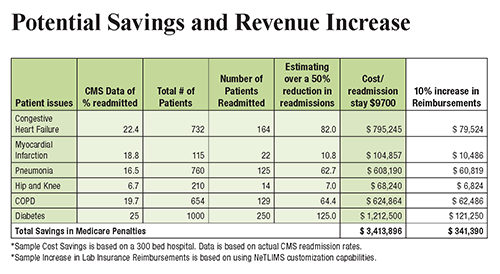
The chart above illustrates how a LIMS offers increased potential to automate processes and scale operations while keeping physicians, patients, and other critical parties up to date. This increase in efficiency and access to data empowers providers to reach improved patient outcomes and reduce hospital readmission rates, increasing revenue for both clinicians and clinical laboratories. (Graphic copyright: NetLIMS.)
When implemented properly, a LIMS also helps laboratories and healthcare facilities meet the terms of MIPS. This reduces Medicare penalties and ensures payment adjustments, which improve revenue streams even further.
Understanding LIMS and Cloud-Based Lab Systems
To help outline and explain the benefits of a LIMS for laboratories and healthcare facilities, The Dark Report, in conjunction with NetLIMS, a global provider of laboratory information management systems to hundreds of hospitals and commercial laboratories worldwide, has produced a free white paper titled, “The Path to More Revenue: Cloud-Based LIMS, Mobile Apps, and Point-of-Care Telehealth.”
- This white paper addresses critical concerns, including:
- Overviews of new technologies;
- The impact of value-based programs on the lab market;
- The importance of MACRA and MIPS adherence;
- How technology, such as a LIMS, can help labs achieve improved efficiency; and,
- Tips on choosing a LIMS vendor to maximize ROI.

To download your free copy of the whitepaper click on this link: Or, copy this URL into your browser: https://www.darkdaily.com/whitepaper/the-path-to-more-revenue-cloud-based-lims-mobile-apps-and-point-of-care-telehealth .
Thanks to advances in LIMS design and development, remote patient digital therapeutics, and cloud-based technology, healthcare providers now have unprecedented opportunities to better manage the health of patients with chronic conditions. In addition, it can help you achieve better efficiency, economics, and compliance with MACRA.
This free white paper is your first step toward significantly reducing hospital readmission rates, bridging the gap between labs, physicians, and other healthcare providers they serve, and positively affecting patient outcomes, improving quality measures, and maximizing reimbursements for all services you provide.
—Jon Stone
Related Information:
The Path to More Revenue: Cloud-Based LIMS, Mobile Apps, and Point-of-Care Telehealth
How Close Is the End of Private Practice Pathology as We’ve Known It?
Attention Anatomic Pathologists: Do You Know Medicare Is Prepared to Change How You Are Paid, Beginning on January 1, 2017?
Innovator Hospitals Bring ICUs into the Info Age, Using New Design Approaches that involve Medical Laboratory Tests










