Nov 26, 2018 | Coding, Billing, and Collections, Instruments & Equipment, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
Despite the widespread adoption of electronic health record (EHR) systems and billions in government incentives, lack of interoperability still blocks potential benefits of digital health records, causing frustration among physicians, medical labs, and patients
Clinical laboratories and anatomic pathology groups understand the complexity of today’s electronic health record (EHR) systems. The ability to easily and securely transmit pathology test results and other diagnostic information among multiple providers was the entire point of shifting the nation’s healthcare industry from paper-based to digital health records. However, despite recent advances, true interoperability between disparate health networks remains elusive.
One major reason for the current situation is that multi-hospital health systems and health networks still use EHR systems from different vendors. This fact is well-known to the nation’s medical laboratories because they must spend money and resources to maintain electronic lab test ordering and resulting interfaces with all of these different EHRs.
Healthcare IT News highlighted the scale of this problem in recent coverage. Citing data from the Healthcare Information and Management Systems Society (HIMSS) Logic database, they note that—when taking into account affiliated providers—the typical health network engages with as many as 18 different electronic medical record (EMR) vendors. Similarly, hospitals may be engaging with as many as 16 different EMR vendors.

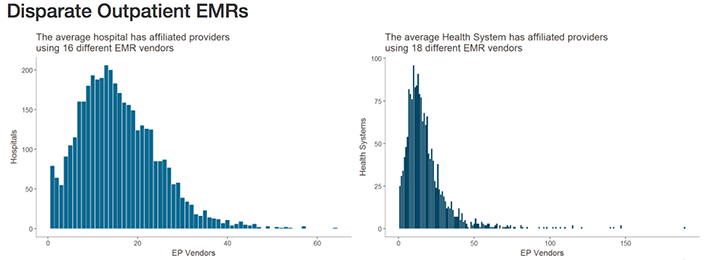
The graphics above illustrates why interoperability is the most important hurdle facing healthcare today. Although the shift to digital is well underway, medical laboratories, physicians, and patients still struggle to communicate data between providers and access it in a universal or centralized manner. (Images copyright: Healthcare IT News.)
The lack of interoperability forces healthcare and diagnostics facilities to develop workarounds for locating, transmitting, receiving, and analyzing data. This simply compounds the problem.
According to a 2018 Physician’s Foundation survey, nearly 40% of respondents identified EHR design and interoperability as the primary source of physician dissatisfaction. It has also been found to be the cause of physician burnout, as Dark Daily reported last year in, “EHR Systems Continue to Cause Burnout, Physician Dissatisfaction, and Decreased Face-to-Face Patient Care.”
Pressure from Technology Giants Fuels Push for Interoperability
According to HITECH Answers, the Centers for Medicare and Medicaid Services (CMS) has paid out more than $38-billion in EHR Incentive Program payments since April 2018.
Experts, however, point out that government incentives are only one part of the pressure vendors are seeing to improve interoperability.
“There needs to be a regulatory push here to play referee and determine what standards will be necessary,” Blain Newton, Executive Vice President, HIMSS Analytics, told Healthcare IT News. “But the [EHR] vendors are going to have to do it because of consumer demand, as things like Apple Health Records gain traction.”
Dark Daily covered Apple’s progress into organizing protected health information (PHI) and personal health records (PHRs) earlier this year in, “Apple’s Update of Its Mobile Health App Consolidates Data from Multiple EHRs and Makes It Easier to Push Clinical Laboratory Data to Patients.” It is one of the latest examples of Silicon Valley tech companies attempting to jump into the health sector and providing patients and consumers access to the troves of medical data created in their lifetime.
Another solution, according to TechTarget, involves developing application programming interfaces (APIs) that allow tech companies and EHR vendors to achieve better interoperability by linking information in a structured manner, facilitating secure data transmission, and powering the next generation of apps that will bring interoperability ever closer to a reality.
TechTarget reported on how University of Utah Hospital’s five hospital/12 community clinic health network, and Intermountain Healthcare, also in Utah, successfully used APIs to develop customized interfaces and apps to improve accessibility and interoperability with their Epic and Cerner EHR systems.
Diagnostic Opportunities for Clinical Laboratories
As consumers gain increased access to their data and healthcare providers harness the current generation of third-party tools to streamline EHR use, vendors will continue to feel pressure to make interoperability a native feature of their EHR systems and reduce the need to rely on HIT teams for customization.
For pathology groups, medical laboratories, and other diagnosticians who interact with EHR systems daily, the impact of interoperability is clear. With the help of tech companies, and a shift in focus from government incentives programs, improved interoperability might soon offer innovative new uses for PHI in diagnosing and treating disease, while further improving the efficiency of clinical laboratories that face tightening budgets, reduced reimbursements, and greater competition.
—Jon Stone
Related Information:
Why EHR Data Interoperability Is Such a Mess in 3 Charts
EHR Incentive Program Status Report April 2018
New FDA App Streamlines EHR Patient Data Collection for Researchers
AAFP Nudges ONC toward EHR Interoperability
A New Breed of Interoperable EHR Apps Is Coming, but Slowly
Top Interoperability Questions to Consider during EHR Selection
EHR Design, Interoperability Top List of Physician Pain Points
2018 Survey of America’s Physicians: Practice Patterns & Perspectives
ONC: 93% of Hospitals Have Adopted Most Recent EHR Criteria, but Most Lag in Interoperability
Open Standards and Health Care Transformation: It’s Finally Delivering on the Value It Promised
Apple’s Update of Its Mobile Health App Consolidates Data from Multiple EHRs and Makes It Easier to Push Clinical Laboratory Data to Patients
EHR Systems Continue to Cause Burnout, Physician Dissatisfaction, and Decreased Face-to-Face Patient Care
Nov 16, 2018 | Digital Pathology, Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing
New study conducted by an international team of researchers suggests that artificial intelligence (AI) may be better than highly-trained humans at detecting certain skin cancers
Artificial intelligence (AI) has been working its way into health technology for several years and, so far, AI tools have been a boon to physicians and health networks. Until now, though, the general view was that it was a supplemental tool for diagnosticians, not a replacement for them. But what if the AI was better at detecting disease than humans, including anatomic pathologists?
Researchers in the Department of Dermatology at Heidelberg University in Germany have concluded that AI can be more accurate at identifying certain cancers. The challenge they designed for their study involved skin biopsies and dermatologists.
They pitted a deep-learning convolutional neural network (CNN) against 58 dermatologists from 17 countries to determine which was more accurate at detecting malignant melanomas—humans or AI. A CNN is an artificial network based on the biological processes that occur when neurons in the brain are connected to each other and respond to what the eye sees.
The CNN won.
“For the first time we compared a CNN’s diagnostic performance with a large international group of 58 dermatologists, including 30 experts. Most dermatologists were outperformed by the CNN. Irrespective of any physicians’ experience, they may benefit from assistance by a CNN’s image classification,” the report noted.
The researchers published their report in the Annals of Oncology, a peer-reviewed medical journal published by Oxford University Press that is the official journal of the European Society for Medical Oncology.

“I expected only a performance on an even level with the physicians. The outperformance even of the average experienced and trained dermatologists was a major surprise,” Holger Haenssle, PhD, Professor of Dermatology at Heidelberg University and one of the authors of the study, told Healthline. Anatomic pathologists will want to follow the further development of this research and its associated diagnostic technologies. (Photo copyright: University of Heidelberg.)
Does AI Tech Have Superior Visual Acuity Compared to Human Eyes?
The dermatologists who participated in the study had varying degrees of experience in dermoscopy, also known as dermatoscopy. Thirty of the doctors had more than five-year’s experience and were considered to be expert level. Eleven of the dermatologists were considered “skilled” with two- to five-year’s experience. The remaining 17 doctors were termed beginners with less than two-year’s experience.
To perform the study, the researchers first compiled a set of 100 dermoscopic images that showed melanomas and benign moles called Nevi. Dermoscopes (or dermatoscopes) create images using a magnifying glass and light source pressed against the skin. The resulting magnified, high-resolution images allow for easier, more accurate diagnoses than inspection with the naked eye.
During the first stage of the research, the dermatologists were asked to diagnose whether a lesion was melanoma or benign by looking at the images with their naked eyes. They also were asked to render their opinions for any needed action, such as surgery and follow-up care based on their diagnoses.
After this part of the study, the dermatologists on average identified 86.6% of the melanomas and 71.3% of the benign moles. More experienced doctors identified the melanomas at 89%, which was slightly higher than the average of the group.
The researchers also showed 300 images of malignant and benign skin lesions to the CNN. The AI accurately identified 95% of the melanomas by analyzing the images.
“The CNN missed fewer melanomas, meaning it had a higher sensitivity than the dermatologists, and it misdiagnosed fewer benign moles as malignant melanoma, which means it had a higher specificity. This would result in less unnecessary surgery,” Haenssle told CBS News.
In a later part of the research, the dermatologists were shown the images a second time and provided clinical information about the patients, including age, gender, and location of the lesion. They were again instructed to make diagnoses and projected care decisions. With the additional information, the doctors’ average detection of melanomas increased to 88.9% and their recognition of benign moles increased to 75.7%. Still below the results of the CNN.
These findings suggest that the visual pattern recognition of AI technology could be a meaningful tool to help physicians and researchers diagnose certain cancers.
“In the future, I think AI will be integrated into practice as a diagnostic aide, particularly in primary care, to support the decision to excise a lesion, refer, or otherwise to reassure that it is benign,” Victoria Mar, PhD, an Adjunct Senior Lecturer in the Department of Public Health and Preventative Medicine at Australia’s Monash University, told Healthline.
“There is the potential for AI technology to be integrated with 2D or 3D skin imaging systems, which means that the majority of benign lesions would be already filtered by the machine, so that we can spend more time concentrating on the difficult or more concerning lesions,” she said. “To me, this means a more productive interaction with the patient, where we can focus on appropriate management and provide more streamlined care.”
AI Performs Well in Other Studies Involving Skin Biopsies
This study is not the only research that suggests entities besides humans may be utilized in diagnosing some cancers from images. Last year, computer scientists at Stanford University performed similar research and found comparable results. For that study, the researchers created and trained an algorithm to visually diagnose potential skin cancers by looking at a database of skin images. They then showed photos of skin lesions to 21 dermatologists and asked for their diagnoses based on the images. They found the accuracy of their AI matched the performance of the doctors when diagnosing skin cancer from viewed images.
And in 2017, Dark Daily reported on three genomic companies developing AI/facial recognition software that could help anatomic pathologists diagnose rare genetic disorders. (See, “Genomic Companies Collaborate to Develop Facial Analysis Technology Pathologists Might Eventually Use to Diagnose Rare Genetic Disorders,” August 7, 2017.)
While many dermatologists read patient biopsies on their own, they also refer high volumes of skin biopsies to anatomic pathologists. A technology that can accurately diagnose skin cancers could potentially impact the workload received by clinical laboratories and anatomic pathology groups.
—JP Schlingman
Related Information:
Dermatologists Hate Him! Meet the Skin-cancer Detecting Robot
Man Against Machine: Diagnostic Performance of a Deep Learning Convolutional Neural Network for Dermoscopic Melanoma Recognition in Comparison to 58 Dermatologists
AI Better than Dermatologists at Detecting Skin Cancer, Study Finds
AI May Be Better at Detecting Skin Cancer than Your Derm
Deep Learning Algorithm Does as Well as Dermatologists in Identifying Skin Cancer
Genomic Companies Collaborate to Develop Facial Analysis Technology Pathologists Might Eventually Use to Diagnose Rare Genetic Disorders
Nov 9, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Pathology
Studies show consumer genealogy databases are much broader than is generally known. If your cousins are in such a database, it’s likely you are too
Recent news stories highlighted crime investigators who used the DNA data in consumer genetic genealogy databases to solve cold cases. Though not widely known, such uses of direct-to-consumer DNA databases is becoming more commonplace, which might eventually lead to requests for clinical laboratories to assist in criminal investigations involving DNA data.
Case in point: investigators found the Golden State Killer, a serial killer/rapist/burglar who terrorized multiple California counties over a dozen years in the 1970s to 1980s, after uploading a DNA sample from the crime scene to GEDmatch, an open-data genomics database that features tools for genealogy research. They made the arrest after discovering a distant relative’s DNA in the genealogy database and matching it to the suspect, CBS News revealed in a 60 Minutes Overtime online report.
These and other investigators are using a technique called familial DNA testing (AKA, DNA Profiling), which enables them to use genetic material from relatives to solve crimes.
Clinical laboratories oversee DNA databases. Could DNA databases—developed and managed over years by medical laboratories for patient care—be subpoenaed by law enforcement investigating crimes?
The question raises many issues for society and for labs, including privacy responsibilities and appropriate use of genetic information. On the other hand, the genetic genie is already out of the bottle.
Leveraging Familia DNA to Solve Crimes a New Trend
“The solving of the Golden State Killer case opened this method up as a possibility, and other crime labs are taking advantage of it. Clearly, a trend has started,” Ruth Dickover, PhD, Director of Forensic Science, University of California, Davis, told the Los Angeles Times.
Indeed, the use of familial DNA testing is moving forward. The Verge reported 19 cold case samples have been identified in recent familial DNA testing and public database searches. It also said two new published studies may propel the technique further.
One study, published in the journal Science, suggests nearly every American of European ancestry may soon be identified through familial DNA testing.
The other study, published in Cell, shows that a person’s relatives can be detected when forensic DNA data are compared with consumer genetic databases.

Noah Rosenberg, PhD (above left), Professor of Population Genetics and Society Biology at Stanford University, is shown above working with Jaehee Kim, PhD (right), a Postdoctoral Research Fellow in Biology, on math that could be used to track down relatives in genealogy databases based on forensic DNA. “This could be a way of expanding the reach of forensic genetics, potentially for solving even more cold cases. But at the same time, it could be exposing participants in those databases to forensic searches they might not have anticipated,” he told Wired. (Photo copyright: Stanford University/L.A. Cicero.)
15 Million People Already in Genealogy Databases
Researchers at Columbia University in New York and Hebrew University of Jerusalem told Science they were motivated by the recent trend of investigations leveraging third-party consumer genomics services to find criminals. But they perceived a gap.
“The big limitation is coverage. And even if you find an individual it requires complex analysis from that point,” Yaniv Erlich, PhD, Associate Professor at Columbia and Chief Science Officer at MyHeritage, told The Verge. MyHeritage is an online genealogy platform.
Others offering consumer genetic testing and family history exploration include 23andMe and Ancestry. As of April 2018, more than 15 million people have participated in direct-to-consumer genetic testing, the researchers noted.
The study aimed to find the likelihood that a person can be identified using a long-range familial search. It included these steps and findings:
- Statistical analysis of 1.28 million people in the MyHeritage database;
- Pairs of people with “identity-by-descent” were removed to avoid bias, such as first cousins and closer relationships;
- Researchers aimed at finding a third cousin or closer relatives for each person in the database;
- 60% of the 1.28 million people were matched with a third cousin or closer relative.
“We project that about 60% of the searches for individuals of European-descent will result in a third cousin or closer match, which can allow their identification using demographic identifiers. Moreover, the technique could implicate nearly any US individual of European descent in the near future,” the researchers wrote.
In an interview with Wired, Erlich added, “The takeaway is it doesn’t matter if you’ve been tested or not tested. You can be identified because the databases already cover such large fractions of the US—at least for European ancestry.”
Matching Forensic and Consumer Genetic Data
Meanwhile, the study published in Cell by researchers at Stanford University, University of California, Davis, and the University of Michigan also suggests investigators could compare forensic DNA samples with consumer genetic databases to find people related to criminals.
That study found:
- 30% to 32% of people in a forensic database could be related to a child or parent in a consumer database;
- 35% to 36% could be tied to a sibling.
These studies reveal that genetic data and familial DNA testing can help law enforcement find suspects, which is a good thing for society. But people who uploaded DNA data to some direct-to-consumer databases may find themselves caught up in searches they do not know about. So may their cousins.
Dark Daily recently covered other similar studies that showed it takes just one person’s DNA to reveal genetic information on an entire family. (See, “The Problems with Ancestry DNA Analyses,” October 18, 2018.) These developments in the use of DNA databases to identify criminals should be an early warning to clinical laboratories building databases of genetic information that, at some future point, law enforcement agencies might want access to those databases as part of ongoing criminal investigations.
—Donna Marie Pocius
Related Information:
Could Your DNA Help Solve a Cold Case?
So Many People Have Had Their DNA Sequenced That They’ve Put Other People’s Privacy in Jeopardy
The DNA Technique That Caught the Golden State Killer is More Powerful than We Thought
Identity Inference of Genomic Data Using Long-Range Familial Searches
Statistical Detection of Relatives Typed with Disjoint Forensic and Biomedical Loci
Genome Hackers Show No One’s DNA is Anonymous Anymore
Stanford Researchers Discover a New Way to Find Relatives from Forensic DNA
The Problems with Ancestry DNA Analyses
Nov 7, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory News, Laboratory Pathology, Laboratory Testing, Management & Operations
CRISPR-Cas9 connection to cancer prompts research to investigate different approaches to gene editing
Dark Daily has covered CRISPR-Cas9 many times in previous e-briefings. Since its discovery, CRISPR, or Clustered Regularly Interspaced Short Palindromic Repeats, has been at the root of astonishing breakthroughs in genetic research. It appears to fulfill precision medicine goals for patients with conditions caused by genetic mutations and has anatomic pathologists, along with the entire scientific world, abuzz with the possibilities such a tool could bring to diagnostic medicine.
All of this research has contributed to a deeper understanding of how cells function. However, as is often the case with new technologies, unforeseen and problematic questions also have arisen.
CRISPR-Cas9 Connection to Cancer
Research conducted at the Wellcome Sanger Institute in the United Kingdom (UK) and published in Nature Biotechnology, examined potential damage caused by CRISPR-Cas9 editing.
“Here we report significant on-target mutagenesis, such as large deletions and more complex genomic rearrangements at the targeted sites in mouse embryonic stem cells, mouse hematopoietic progenitors, and a human differentiated cell line,” wrote the authors in their introduction.
Another study, this one conducted by biomedical researches at Cambridge, Mass., and published in Nature, describes possible toxicity caused by Cas9.
“Our results indicate that Cas9 toxicity creates an obstacle to the high-throughput use of CRISPR-Cas9 for genome engineering and screening in hPSCs [human pluripotent stem cells]. Moreover, as hPSCs can acquire P53 mutations, cell replacement therapies using CRISPR-Cas9-enginereed hPSCs should proceed with caution, and such engineered hPSCs should be monitored for P53 function.”
Essentially what both groups of researchers found is that CRISPR-Cas9 cuts through the double helix of DNA, which the cell responds to as it would any injury. A gene called p53 then directs a cellular “first-aid kit” to the “injury” site that either initiates self-destruction of the cell or repairs the DNA.
Therefore, in some instances, CRISPR-Cas9 is inefficient because the repaired cells continue to function. And, the repair process involves the p53 gene. P53 mutations have been implicated in ovarian, colorectal, lung, pancreatic, stomach, liver, and breast cancers.
Though important, some experts are downplaying the significance of the findings.

Erik Sontheimer, PhD (above), Professor, RNA Therapeutics Institute, at the University of Massachusetts Medical School, told Scientific American that the two studies are important, but not show-stoppers. “This is something that bears paying attention to, but I don’t think it’s a deal-breaker,” he said. (Photo copyright: University of Massachusetts.)
“It’s something we need to pay attention to, especially as CRISPR expands to more diseases. We need to do the work and make sure edited cells returned to patients don’t become cancerous,” Sam Kulkarni, PhD, CEO of CRISPR Therapeutics, told Scientific American.
Both studies are preliminary. The implications, however, is in how genes that have become corrupted are used.
“It is unclear if the findings translate into cells actually used in clinical studies,” Bernhard Schmierer, PhD, co-author of a paper titled, “CRISPR-Cas9 Genome Editing Induces a p53-mediated DNA Damage Response,” told Scientific American.
Nevertheless, the cancer-cat is out of the bag.
Targeting RNA Instead of DNA with CRISPR-Cas13d
A team from the Salk Institute may have found a solution. They are investigating a different enzyme—Cas13d—which, in conjunction with CRISPR would target RNA rather than DNA. “DNA is constant, but what’s always changing are the RNA messages that are copied from the DNA. Being able to modulate those messages by directly controlling the RNA has important implications for influencing a cell’s fate,” Silvana Konermann, PhD, a Howard Hughes Medical Institute (HHMI) Hanna Gray Fellow and member of the research team at Salk, said in a news release.
The Salk team published their findings in the journal Cell. The paper describes how “scientists from the Salk Institute are reporting for the first time the detailed molecular structure of CRISPR-Cas13d, a promising enzyme for emerging RNA-editing technology. They were able to visualize the enzyme thanks to cryo-electron microscopy (cryo-EM), a cutting-edge technology that enables researchers to capture the structure of complex molecules in unprecedented detail.”
The researchers think that CRISPR-Cas13d may be a way to make the process of gene editing more effective and allow for new strategies to emerge. Much like how CRISPR-Cas9 led to research into recording a cell’s history and to tools like SHERLOCK (Specific High-sensitivity Enzymatic Reporter unLOCKing), a new diagnostic tool that works with CRISPR and changed clinical laboratory diagnostics in a foundational way.
Dark Daily reported on this breakthrough last year. (See, “CRISPR-Related Tool Set to Fundamentally Change Clinical Laboratory Diagnostics, Especially in Rural and Remote Locations,” August 4, 2017.)
Each discovery will lead to more branches of inquiry and, hopefully, someday it will be possible to cure conditions like sickle cell anemia, dementia, and cystic fibrosis. Given the high expectations that CRISPR and related technologies can eventually be used to treat patients, pathologists and medical laboratory professionals will want to stay informed about future developments.
—Dava Stewart
Related Information:
Repair of Double-Strand Breaks Induced by CRISPR-Cas9 Leads to Large Deletions and Complex Rearrangements
P53 Inhibits CRISPR-Cas9 Engineering in Human Pluripotent Stem Cells
CRISPR-Edited Cells Linked to Cancer Risk in 2 Studies
CRISPR-Cas9 Genome Editing Induces a p53-Mediated DNA Damage Response
Decoding the Structure of an RNA-Based CRISPR System
Structural Basis for the RNA-Guided Ribonuclease Activity of CRISPR-Cas13d
CRISPR Timeline
What Are Genome Editing and CRISPR-Cas9?
Federal Court Sides with Broad in CRISPR Patent Dispute
Top Biologists Call for Moratorium on Use of CRISPR Gene Editing Tool for Clinical Purposes Because of Concerns about Unresolved Ethical Issues
CRISPR-Related Tool Set to Fundamentally Change Clinical Laboratory Diagnostics, Especially in Rural and Remote Locations
Researchers at Several Top Universities Unveil CRISPR-Based Diagnostics That Show Great Promise for Clinical Laboratories
Nov 5, 2018 | Digital Pathology, Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing
Computer-assisted analysis using Google’s LYNA algorithm shows significant gains in speed required to analyze stained lymph node slides and sensitivity of micrometastases detection in two recent studies
Anatomic pathologists understand the complexities of reviewing slides and samples for signs of cancer’s spread. Two studies involving a new artificial intelligence (AI) algorithm from Google (NASDAQ:GOOGL) claim their “deep learning” LYmph Node Assistant (LYNA) provides increases to both the speed at which pathologists can analyze slides and improved accuracy in detecting metastatic breast cancer within the slide samples used for the studies.
Google’s first study was published in the Archives of Pathology and Laboratory Medicine and investigated the accuracy of the algorithm using digital pathology slides. Google’s second study, published in The American Journal of Surgical Pathology, looked at how pathologists might harness the algorithm to improve workflows and use the tool in a clinical setting.
Medical laboratories and other diagnostics providers are already familiar with the improvement potential of automation and other technology-based approaches to diagnosis and analysis. Google’s LYNA is an example of how AI and machine learning improvements can serve as a supplement to—not a replacement for—the skills of experts at pathology groups and clinical laboratories.
Early research done by Google indicates that integrating LYNA into existing workflows could allow pathologists to spend less time analyzing slides for minute details. Instead, they could focus on other more challenging tasks while the AI analyzes gigapixels worth of slide data to highlight regions of concern in slides and samples for deeper manual inspection.
LYNA Achieves 99% Accuracy in Study of Metastatic Breast Cancer Detection
According to the research cited in a Google AI Blog post, roughly 25% of metastatic lymph node staging classifications would change if subjected to a second pathologic review. They further note that when faced with time constraints, detection sensitivity for small metastases on individual slides can be as low as 38%.
In findings published in Archives of Pathology and Laboratory Medicine, Google researchers analyzed whole slide images from hematoxylin-eosin-stained lymph nodes for 399 patients sourced from the Camelyon16 challenge dataset. Of those slides, researchers used 270 to train LYNA and the remaining 129 for analysis. They then compared the LYNA findings to those of an independent lab using a different scanner.
“LYNA achieved a slide-level area under the receiver operating characteristic (AUC) of 99% and a tumor-level sensitivity of 91% at one false positive per patient on the Camelyon16 evaluation dataset,” the researchers stated. “We also identified [two] ‘normal’ slides that contained micrometastases.”
Google’s algorithm later received an AUC of 99.6% on a secondary dataset.
“Artificial intelligence algorithms can exhaustively evaluate every tissue patch on a slide, achieving higher tumor-level sensitivity than, and comparable slide-level performance to, pathologists,” the researchers continued. “These techniques may improve the pathologist’s productivity and reduce the number of false negatives associated with morphologic detection of tumor cells.”
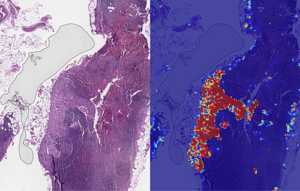
Left: sample view of a slide containing lymph nodes, with multiple artifacts: the dark zone on the left is an air bubble, the white streaks are cutting artifacts, the red hue across some regions are hemorrhagic (containing blood), the tissue is necrotic (decaying), and the processing quality was poor. Right: LYNA identifies the tumor region in the center (red), and correctly classifies the surrounding artifact-laden regions as non-tumor (blue). (Image and caption copyright: Google AI Blog.)
Faster Analysis through Software Assistance
Rapid diagnosis helps improve cancer outcomes. Yet, manually reviewing and analyzing complex digital slides is time-consuming. Time constraints might also lead to false negatives due to micrometastases or small suspicious regions that slip by pathologists undetected.
The Google research team of the study published in The American Journal of Surgical Pathology sought to gauge the impact LYNA might have on the histopathologic review of lymph nodes for trained pathologists. In their multi-reader multi-case study, researchers analyzed differences in both sensitivity of detecting micrometastases and the average review time per image using both computer-aided detection and unassisted detection for six pathologists across 70 slides.
Using the LYNA algorithm to identify and outline regions likely to contain tumors, the researchers found that sensitivity increased from 83% to 91%. The time to review slides also saw a significant reduction from 116 seconds in the unassisted mode to 61 seconds in the assisted mode—a time savings of roughly 47%.
“Although some pathologists in the unassisted mode were less sensitive than LYNA,” the researchers stated, “all pathologists performed better than the algorithm alone in regard to both sensitivity and specificity when reviewing images with assistance.”
The Future of Digital Pathology using LYNA
While the two studies show positive results, both studies also reveal shortcomings. Google highlighted both limited dataset sizes and simulated diagnostic workflows as potential concerns and areas on which to focus future studies.
Still, Google’s researchers believe that algorithms such as LYNA will help to power the future of diagnostics as healthcare in the digital era continues to mature. “We remain optimistic,” state the authors of the Google AI Blog post, “that carefully validated deep learning technologies and well-designed clinical tools can help improve both the accuracy and availability of pathologic diagnosis around the world.”
While other industries see risk in the growth of AI, both studies performed by researchers at Google show how computer-assisted workflows and machine learning could accentuate and bolster the skills of trained diagnosticians, such as anatomic pathologists and clinical laboratory technicians. By working to compensate for weak points in both human skill and computer reasoning, the outcome could be greater than either AI or humans can achieve separately.
—Jon Stone
Related Information:
Google Creates AI to Detect When Breast Cancer Spreads
Google Deep Learning Tool 99% Accurate at Breast Cancer Detection
Google’s AI Software Seeks to Detect Advanced Breast Cancer Better Than We Have Before
Google’s AI Is Better at Spotting Advanced Breast Cancer than Pathologists
Google AI Claims 99% Accuracy in Metastatic Breast Cancer Detection
Applying Deep Learning to Metastatic Breast Cancer Detection
Assisting Pathologists in Detecting Cancer with Deep Learning
Diagnostic Assessment of Deep Learning Algorithms for Detection of Lymph Node Metastases in Women with Breast Cancer
Nov 2, 2018 | Coding, Billing, and Collections, Compliance, Legal, and Malpractice, Digital Pathology, Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
Patient privacy, ethics of monetizing not-for-profit data, and questions surrounding industry conflicts appear after the public announcement of an arrangement to grant exclusive access to academic pathology slides and samples
Clinical laboratories and anatomic pathology groups already serve as gatekeepers for a range of medical data used in patient treatments. Glass slides, paraffin-embedded tissue specimens, pathology reports, and autopsy records hold immense value to researchers. The challenge has been how pathologists (and others) in a not-for-profit academic center could set themselves up to potentially profit from their exclusive access to this archived pathology material.
Now, a recent partnership between Memorial Sloan Kettering Cancer Center (MSK) and Paige.AI (a developer of artificial intelligence for pathology) shows how academic pathology laboratories might accomplish this goal and serve a similar gatekeeper role in research and development using the decades of cases in their archives.
The arrangement, however, is not without controversy.
New York Times, ProPublica Report
Following an investigative report from the New York Times (NYT) and ProPublica, pathologists and board members at MSK are under fire from doctors and scientists there who have concerns surrounding ethics, exclusivity, and profiting from data generated by physicians and but owned by MSK.
“Hospital pathologists have strongly objected to the Paige.AI deal, saying it is unfair that the founders received equity stakes in a company that relies on the pathologists’ expertise and work amassed over 60 years. They also questioned the use of patients’ data—even if it is anonymous—without their knowledge in a profit-driven venture,” the NYT article states.
Prominent members of MSK are facing scrutiny from the media and peers—with some relinquishing stakes in Paige.AI—as part of the backlash of the report. This is an example of the perils and PR concerns lab stakeholders might face concerning the safety of data sharing and profits made by medical laboratories and other diagnostics providers using patient data.
Controversy Surrounds Formation of Paige.AI/MSK Partnership
In February 2018, Paige.AI announced closing the deal on a $25-million round of Series A funding, and in gaining exclusive access to 25-million pathology slides and computational pathology intellectual property held by the Department of Pathology at Memorial Sloan Kettering. Coverage by TechCrunch noted that while MSK received an equity stake as part of the licensing agreement, they were not a cash investor.
Creation of the company involved three hospital insiders and three additional board members with the hospital itself established as part owner, according to STAT.
Unnamed officials told the NYT that board members at MSK only invested in Paige.AI after earlier efforts to generate outside interest and investors were unsuccessful. NYT’s coverage also notes experts in non-profit law and corporate governance have raised questions as to compliance with federal and state laws that govern nonprofits in light of the Paige.AI deal.
Growing Privacy Fallout and Potential Pitfalls for Medical Labs
The original September 2018 NYT coverage noted that Klimstra intends to divest his ownership stake in Paige.AI. Later coverage by NYT in October, notes that Democrat Representative Debbie Dingell of Michigan submitted a letter questioning details about patient privacy related to Paige.AI’s access to MSK’s academic pathology resources.
Privacy continues to be a focus for both media and regulatory scrutiny as patient data continues to fill electronic health record (EHR) systems as well as research and commercial databases. Dark Daily recently covered how University of Melbourne researchers demonstrated how easily malicious parties might reidentify deidentified data. (See “Researchers Easily Reidentify Deidentified Patient Records with 95% Accuracy; Privacy Protection of Patient Test Records a Concern for Clinical Laboratories”, October 10, 2018.)
According to the NYT, MSK also issued a memo to employees announcing new restrictions on interactions with for-profit companies with a moratorium on board members investing in or holding board positions in startups created within MSK. The nonprofit further noted it is considering barring hospital executives from receiving compensation for their work on outside boards.
However, MSK told the NYT this only applies to new deals and will not affect the exclusive deal between Paige.AI and MSK.
“We have determined,” MSK wrote, “that when profits emerge through the monetization of our research, financial payments to MSK-designated board members should be used for the benefit of the institution.”
There are no current official legal filings regarding actions against the partnership. Despite this, the arrangement—and the subsequent fallout after the public announcement of the arrangement—serve as an example of pitfalls medical laboratories and other medical service centers considering similar arrangements might face in terms of public relations and employee scrutiny.
Risk versus Reward of Monetizing Pathology Data
While the Paige.AI situation is only one of multiple concerns now facing healthcare teams and board members at MSK, the events are an example of risks pathologists take when playing a role in a commercial enterprise outside their own operations or departments.
In doing so, the pathologists investing in and shaping the deal with Paige.AI brought criticism from reputable sources and negative exposure in major media outlets for their enterprise, themselves, and MSK as a whole. The lesson from this episode is that pathologists should tread carefully when entertaining offers to access the patient materials and data archived by their respective anatomic pathology and clinical laboratory organizations.
—Jon Stone
Related Information:
Sloan Kettering’s Cozy Deal with Start-Up Ignites a New Uproar
Paige.AI Nabs $25M, Inks IP Deal with Sloan Kettering to Bring Machine Learning to Cancer Pathology
Sloan Kettering Executive Turns Over Windfall Stake in Biotech Start-Up
Cancer Center’s Board Chairman Faults Top Doctor over ‘Crossed Lines’
Memorial Sloan Kettering, You’ve Betrayed My Trust
LVHN Patient Data Not Shared with For-Profit Company in Sloan Kettering Trials
Researchers Easily Reidentify Deidentified Patient Records with 95% Accuracy; Privacy Protection of Patient Test Records a Concern for Clinical Laboratories










