Mar 23, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory Pathology, Management & Operations
Consumer demand for health trackers combined with other smartwatch capabilities is driving a trend away from simple health trackers and toward more complex devices, such as the Apple Watch, for their more powerful capabilities
It is still an open question as to whether clinical laboratories will experience an onrush of patient test data streaming at them from healthcare consumer portals and mobile devices. The popularity of wearable fitness/medical technology has been widely touted in the media. Predictions have been that these devices—when coupled with smartphone and tablet applications (apps)—would generate substantial volumes of digital patient data that would be useful for medical laboratories to capture and add to the clinical lab test data of the patients they serve.
But will these predictions of a flood of data from wearable devices become reality? Is this a trend about which medical laboratories should be concerned? Recent statistics provide some insight into these questions. For example, the sales numbers for wearable devices are significant.
Smartwatches Gaining Ground in Wearable Fitness Market
In 2016, 102.4 million wearable devices were sold, which was a 25% increase over the previous year, according to Smart Insights, a publisher for marketers. Now, several sports apparel companies, such as Adidas and Under Armour, are either launching smartwatches with health/fitness-related software and activity trackers, or eliminating their digital fitness business units altogether.
And according to MobiHealthNews, “[today’s] landscape looks awfully different.
“I think the industry is still struggling to find real, meaningful points of reference with consumers,” Dan Ledger, Principal and Founder, Path Collaborative, a Massachusetts consulting firm, told MobiHealthNews. “You hear anecdotes of people who had Fitbit (NYSE:FIT) and lost weight. But it hasn’t really been a success as a market product like a smartphone—like a lot of these companies were expecting when they were reading the tea leaves four or five years ago.”
For example, Adidas reassigned employees working in the fitness watch and sensor-enabled footwear departments to other areas, according to the Portland Business Journal. “We are integrating digital across all areas of our business and will continue to grow our digital expertise but in a more integrated way,” an Adidas spokesperson told Just-Style.
And, Nike announced its intention late last year to abandon the wearables market altogether. “It wasn’t authentic to who we were,” Jordan Rice, Senior Director of Nike NXT Smart Systems Engineering, told MobiHealthNews.
Meanwhile, Under Armour announced in 2017 that it planned to eliminate the UA HealthBox, a wearable device that offered a connected activity tracker, heart rate monitor, and smart scale tools, according to MHealth Spot. Instead, the publication reported, Under Armour was partnering with Samsung on fitness apps:
- MyFitnessPal;
- MapMyFitness;
- Endomondo; and,
- UA Record.
More Consumers Strapping on Smartwatches
Fitbit recently released the Fitbit Ionic Watch. According to Fitbit’s website, features include:
- Personal coaching;
- Heart rate monitor;
- All-day activity tracking;
- Sleep stages monitoring; and more.

The smartwatch may be the new “smart” way to go, compared to simple activity trackers. Smartwatch manufactures are partnering with biometric monitoring app developers (such as Apple Watch and IBM Watson Health, shown above) to service consumers who need to monitor, capture, and distribute their critical health data. (Photo copyright: Alexey Boldin/Shutterstock.)
Consumer Reports, citing NPD Group market data, noted smartwatches are increasingly becoming the device-of-choice for consumers who gather fitness data. Besides tracking heart rate, some smartwatch apps also release notifications about accomplishment of goals, enable access to e-mail, and more.
Consumer Reports noted:
- Smartwatches were used by 17% of US adults in the first quarter of 2015, and the remaining 83% in the demographic used activity trackers;
- Smartwatch use jumped to 38% by the fourth quarter of 2017; and,
- Smartwatches will rise to 48% of new market purchases by the fourth quarter this year.
Hardware is Hard
Fitness wearable devices have long been touted by the media for their potential to stream critical health data directly to physicians, to patients’ electronic health records, and to medical laboratories. Dark Daily foresaw in 2016 that, when paired with a smartphone or table computer, the momentum of the fitness wearables trend was substantial. For this reason, clinical laboratory managers and pathologists would want to stay current with these developments. However, today it appears companies offering wearable monitoring devices could be finding it more difficult than anticipated to capture the attention of consumers and leverage what the devices do.
In the end, sports apparel companies are not leaving the digital fitness space entirely, but simply adjusting to new consumer demands. Clinical laboratory leaders will want to keep watch on these developments as the trend evolves. The outcome could alter how patient data enters the pathology workflow.
—Donna Marie Pocius
Related Information:
Digital Marketing Strategy Wearables Statistics 2017
Sports Apparel Brands are All Walking Away from Fitness Wearables
Under Armour Kills the HealthBox Suite of Connected Devices
Adidas to Cut Digital Sports Division
Fitness Tracker or Smartwatch: Which is Best for You?
Improvements to Fitness Wearables Help Stream Data from Consumers Homes to EHRs and Clinical Pathology Laboratories
Mar 21, 2018 | Digital Pathology, Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Sales and Marketing, Laboratory Testing, News From Dark Daily
January’s press release confirmed the tech company is working to integrate critical medical data into its mobile devices, while further promoting interoperability and patient access
While interoperability has improved since the earliest electronic health record (EHR) systems, today’s active patients often need to sort through multiple healthcare portals—including those of clinical laboratories and anatomic pathology groups—to get a comprehensive view of their medical history. Not only can this be time consuming, but also inconvenient if the patient lacks access to a computer.
Thus, it’s no surprise that in a January 24 press release, mobile technology giant Apple announced plans to enter the development ring and create an improved EHR for its mobile device users by updating its existing “Health” mobile application (app). The iOS 11.3 update, among other things, is designed to enable Apple iPhone owners to receive critical medical data, such as medical laboratory test results, directly on their devices.
“Our goal is to help consumers live a better day. We’ve worked closely with the health community to create an experience everyone has wanted for years—to view medical records easily and securely right on your iPhone,” said Apple COO Jeff Williams in the press release.

Jeff Williams (above), COO at Apple, notes that, “By empowering customers to see their overall health, we hope to help consumers better understand their health and help them lead healthier lives.” (Photo copyright: Apple.)
The new features are already available to developers in the latest iOS 11.3 beta 3 release. However, release to the public is expected soon with the issuance of the iOS 11.3 final release. This means that patients will not need to download extra apps—or remember to use them—to take advantage of the feature.
New Way to Improve Patients’ Access to Health Data or Just Another Data Silo?
The Apple Health Records platform adheres to Fast Healthcare Interoperability Resources (FHIR) protocols for transmission of data. Providers send information to Apple which then aggregates the information, transmits it to patients’ iPhones and notifies them of the updates.
All information stored on the device is encrypted in storage and protected from unauthorized access by the user’s password.
Through the new Health Records interface, users view this aggregated data as a timeline, conduct searches, and share information with other parties as they deem appropriate.
Current medical information listed in the press release includes:
- Allergies;
- Conditions;
- Immunizations;
- Clinical laboratory results;
- Medications;
- Procedures; and,
- Vitals.
Currently, the platform integrates data from three major EHR developers:
- Epic;
- Cerner; and,
- AthenaHealth
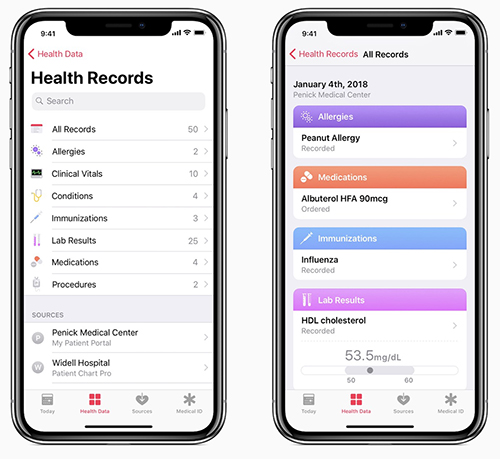
Apple’s update to the Health app makes it easier for people to access and control of all of their health records and data. This included medical laboratory tests. (Image and caption copyright: Apple.)
Apple is also working with 12 health institutions across the US in the first phase of the project, including:
In the Apple press release, Stephanie Reel, CIO at John Hopkins Medicine in Baltimore, stated, “Streamlining information sharing between patients and their caregivers can go a long way towards making the patient experience a positive one. This is why we are excited about working with Apple to make accessing secure medical records from an iPhone as simple for a patient as checking email.”
Previous Attempts at Mobile Health Record Devices Got Mixed Results
This isn’t the first time a major technology company has attempted to enter the mobile health market. Google Health was shuttered in 2011 citing low adoption. Wearable fitness trackers, such as Fitbit (NYSE:FIT) enjoyed a bubble, but are now seeing mixed success in terms of long-term adoption and use, according to The Motley Fool. More to the point, they’ve never quite become the holy grail of monitoring and data collection that some experts predicted, Huffington Post reported.
However, Apple’s investments and interest in healthcare-related technologies has led to wide speculation that they would enter the health market this year. (See Dark Daily “Apple May Be Developing Mobile Device Technology to Monitor User’s Health and Transmit Data in Real Time.”)
Larry Dignan, Editor-in-Chief at ZDNet, builds a compelling case for why this could be the attempt that succeeds in providing a consolidated platform for clinical laboratories, physicians, and other care providers to push data directly to patients and—with the patient’s permission—to each other, regardless of the platforms healthcare facilities use to store and transmit data.
He notes that much of Apple’s newest features build on foundations laid by the healthcare industry to create scalable, functional EHR systems. By working with existing protocols, Apple’s Health Records platform is already positioned for compatibility with many healthcare providers.
Furthermore, Apple is already known for partnering at the enterprise level with major businesses and industries, while also holding the trust of millions of Americans who store their personal information on Apple devices.
Is Apple the Future of EHRs?
Despite this, until the platform—and adoption by the public—is proven a success, it will be yet another walled garden of medical information. Even then, Apple is only one segment of the global mobile market.
Unless Apple provides access to other platforms (such as Android), those patients—and the medical communities serving them—are left consolidating information on their own through a sprawl of various portals. This also means that medical laboratories, pathology groups, and other service providers must continue to invest time and funding into communicating data in ways compatible with a plethora of internal and external systems and software.
Still, the platform offers an intriguing glimpse at the future of medical records and heralds a shift toward empowering patients with easy, comprehensive access to their own data, which would be a boon to the medical laboratory industry.
—Jon Stone
Related Information:
Apple Previews iOS 11.3
Apple Announces Effortless Solution Bringing Health Records to iPhone
With Medical Records Tools, Apple Wades Deeper into Digital Health
Apple Confirms “Health Records” Solution with Aim to Bring Medical Records to iPhone
Apple Will Let You Keep Your Medical Records on Your iPhone
Apple Unveils mHealth Integration with EMR Data through Health App
Apple, Inc. Wants to Solve the Problem of Electronic Health Records
Viewpoint: How Realistic Is Apple’s Attempt at the EHR Industry? Very—6 Reasons Why
Apple Can Win Electronic Medical Record Game with Health Records in iOS 11.3: Here’s 7 Reasons Why
Apple Is Officially in the EHR Business. Now What?
Apple to Launch Health Records App with HL7’s FHIR Specifications at 12 Hospitals
Could Amazon or Apple Actually Make a Dent in the EHR Market?
Apple May Be Developing Mobile Device Technology to Monitor User’s Health and Transmit Data in Real Time
Mar 12, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing
Public health agencies and physicians would gain access to accurate, rapid dip-stick test that could give results similar to a pregnancy test
Tuberculosis is a major killer that ranks alongside HIV/AIDS as a leading cause of death worldwide. This deadly disease takes the lives of more than a million people each year. And, unfortunately, traditional medical laboratory testing using X-rays, blood/skin/sputum specimens, or the new molecular diagnostic systems can be time consuming and expensive.
Now, scientists at George Mason University (GMU) in Virginia have developed a urine test for tuberculosis (TB) that could lead to a dip-stick technology that would accurately and rapidly diagnose the deadly lung disease. Similar to a pregnancy test, if successfully developed for use in clinical settings, the dip-stick could not only enable public health agencies to test for TB more effectively, but also allow primary care physicians and other doctors to easily test their patients for TB at the point of care. However, it also could mean clinical laboratories might find their participation.
Nearly All TB Deaths Occur in Resource Strapped Areas
Such a breakthrough would certainly be a boon to public health and global healthcare, especially in resource strapped areas of the world. According to the World Health Organization (WHO), more than 95% of the 1.7 million TB deaths each year occur in low- and middle-income countries. This is one reason why an inexpensive and easy-to-use detection method for diagnosing the lung disease has long been sought. TB is curable, particularly if diagnosed early.
With that goal in mind, an international team led by Alessandra Luchini, PhD, Associate Professor at GMU, and Lance Liotta, PhD, MD, co-director and co-founder of the GMU Center for Applied Proteomics and Molecular Medicine, developed the potentially revolutionary urine test that uses nanotechnology to measure a sugar molecule in urine that identifies TB with a high degree of accuracy. The scientists published their results in Science Translational Magazine.

George Mason University scientists Alessandra Luchini, PhD (above left), and Lance Liotta, MD, PhD (above right), head an international team that has developed a nanotechnology that may lead to a simple dipstick urine test to detect tuberculosis. Such a test could greatly impact medical laboratories by reducing the need for traditional lab tests. (Photo copyrights: George Mason University.)
While past attempts at developing an accurate urine test for TB failed to reliably detect low concentrations of the sugar entity lipoarabinomannan (LAM) in HIV-negative, TB-infected patients, the GMU team developed a technology capable of doing so.
According to New Scientist, the GMU team’s test “uses tiny molecular cages embedded with a special dye that can catch and trap these sugar molecules. This makes the test capable of detecting the sugar at low concentrations, making the technique as much as 1,000 times more accurate [than] previous methods for detecting TB in urine.”
“We can measure now what could never be measured before,” Liotta noted in a news release.
World Health Organization Recommends Not Using Serodiagnostic Blood Tests
Common methods to detect TB currently include microscopy of sputum samples—a fast and accurate but expensive detection method (that also can diagnosis drug resistant disease)—or a skin test. A third test for TB, an Interferon-Gamma Release Assay, provides results in less than 24 hours but cannot distinguish between active and latent infection. In 2011, the WHO issued a Policy Recommendation urging countries to stop using serodiagnostic blood tests to diagnose TB, calling these tests unreliable and inaccurate. X-rays, meanwhile, detect only advanced lung damage.
In the GMU study, 48 Peruvian patients were chosen, all with active pulmonary TB, none of whom was infected with HIV or previously had received treatment for TB. According to the research, TB infections were detected with greater than 95% sensitivity, with TB-positive, HIV-negative patients having detectably higher concentrations of LAM in their urine compared to the controls. Patients who had more advanced disease also had elevated LAM concentrations. Eight of nine patients who were smear-negative and culture-positive for TB tested positive for urinary LAM.
“The technology can be configured in a variety of formats to detect a panel of previously undetectable very-low-abundance TB urinary analytes … This technology has broad implications for pulmonary TB screening, transmission control, and treatment management for HIV-negative patients,” the study’s authors told Science.
While The Scientist reports the GMU urine test gives results in about 12 hours, Luchini’s goal is for that timeframe to be dramatically shortened as the test is refined.
“We showed that our technology could be used to measure several different kinds of markers for TB in the urine and could be configured as a rapid test similar to a pregnancy test,” Luchini said in a GMU news release.
According to the university, GMU researchers will continue their work in Peru, where students will begin testing hundreds of patients as part of a research study. Grants from the National Institutes of Health and the Bill and Melinda Gates Foundation are funding their work. Luchini told New Scientist her goal is to make their TB urine test easier to use and to test it on thousands more people.
If successful, she predicts the test could be commercially available for physicians and clinical laboratories to use within three years. GMU’s biotechnology partner Ceres Nanosciences will be commercializing the technology, with the aim of making the test available worldwide, the university said in a statement.
—Andrea Downing Peck
Related Information:
TB, or Not TB? At Last, a Urine Test Can Diagnose It Quickly
Urine Lipoarabinomannan Glycan in HIV-Negative Patients with Pulmonary Tuberculosis Correlates with Disease Severity
Mason Scientists Develop Nanotechnology-based Urine Test that Could Lead to Early TB Detection
Urine Test for TB Yields Results in 12 Hours
Tuberculosis Serodiagnostic Tests Policy Statement 2011
Tuberculosis Mortality Nearly Halved Since 1990
Tuberculosis Fact Sheet
Mar 7, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory Pathology
High-flying service expected to take flight in other African countries this year, as Tanzania announces launch of ‘world’s largest’ drone network for medical supply and clinical laboratory specimen deliveries
Anatomic pathologists and medical laboratories know that blood is a scarce and life-saving commodity. This is especially true in developing countries. Rising to that need, a California-based logistics company is using drones to provide on-demand access to vital blood supplies in Rwanda and Tanzania, creating a so-called “Uber for blood” delivery service that is poised for deployment to other regions in Africa as well.
Silicon Valley Start-up Zipline is an American automated logistics company based in California that transported more than 5,500 units of blood in 2017 to 12 regional hospitals from a base in the east of Rwanda, reported The Guardian.
The high-flying Silicon Valley startup began operating in the African nation in October 2016 and has cut blood delivery time from four hours to an average of about 30 minutes! Shipments arrive by parachute, dropping within a narrow target landing zone.
Though the Rwandan government, which is funding the project, has not officially released data quantifying the drones’ impact, the fixed-wing drones (called Zips) have been credited with reducing deaths due to malaria, childbirth, and other medical emergencies.
World’s Largest Drone Delivery Network
In August, Zipline announced plans to launch what it claims is the world’s largest drone delivery network in partnership with the government of Tanzania, a country of 56 million people. Zipline will open four distribution centers in the country during the next four years, with the first drones taking to the air in the first quarter of 2018 from the Tanzanian capital city of Dodoma, according to Cargo Forwarder Global (CFG), a cargo and logistics industry news website.
“Every life is precious. The government of Tanzania through the Ministry of Health, Community Development, Gender, Elderly and Children, has made great achievements in improving health services including the availability of medicines in all public health facilities,” Mpoki Ulisubisya, MD, Permanent Secretary of the Tanzania Ministry of Health, stated in a press release. “Our vision is to have a healthy society with improved social well-being that will contribute effectively to personal and national development; working with Zipline will help make that vision a reality.”

Zipline co-founder and CEO Keller Rinaudo (above) speaking at a 2017 TEDTalk on partnering with Rwanda and Tanzania to deliver life-saving medical supplies—including blood and medical laboratory products—by drone up to 500 times each day. Click on image above to view video. (Photo copyright: TEDTalk.)
Each of the four distribution centers will be equipped with up to 30 drones, capable of making 500 on-demand delivery flights per day. Drones will provide access to more than 1,000 clinics, many of which lack well-functioning supply chains due to surface roads that “become increasingly bumpy and eventually impassable, particularly during the rainy season,” the CFG website reported.
“We strive to ensure that all 5,640 public health facilities have all the essential medicines, medical supplies, and laboratory reagents they need, wherever they are—even in the most hard-to-reach areas,” Laurean Bwanakunu, Director General of Tanzania’s Medical Stores Department, said in a news release. “But that mission can be a challenge during emergencies, times of unexpected demand, bad weather, or for small but critical orders. Using drones for just-in-time deliveries will allow us to provide health facilities with complete access to vital medical products no matter the circumstance.”
According to Zipline, health workers at remote clinics and hospitals text orders to Zipline for the medical products they need, which are stored in distribution centers. Within minutes, a Zipline Distribution Center can pack and prepare blood and other stored medical products for the 60-mph flight to any delivery site within a 93-mile round trip.

A Zipline worker technician launches a drone in Muhanga. (Photo copyright: Stephanie Aglietti/AFP/Getty Images. Caption copyright: The Guardian.)
In a CFG statement, Keller Rinaudo, Zipline’s co-founder and Chief Executive Officer, said the expansion to Tanzania will make East Africa a world leader in drone logistics.
“Millions of people across the world die each year because they can’t get the medicine they need when they need it,” noted Rinaudo. “It’s a problem we can help solve with on-demand drone delivery. Now African nations are showing the world how it’s done.”
Rinaudo told IEEE Spectrum that Zipline expects to expand its operations to several other countries in 2018, though he did not specify where Zips will be flying next. He said the next generation of Zips will be able to fly longer distances, carry larger payloads, and make more deliveries per day.
“Rwanda has shown such remarkable success that a lot of other countries want to follow in its footsteps,” noted Rinaudo. “The problems we’re solving in Rwanda aren’t Rwanda problems, they’re global problems—rural healthcare is a challenge everywhere.”
Drones Delivering Medical Laboratory Specimens Globally
Africa is not the only part of the world where drones are taking flight to deliver blood and medical laboratory specimens. Johns Hopkins University Medicine researchers set a record in America for the longest distance drone delivery of viable medical specimens when its test drone traveled 161 miles across the Arizona desert, an event that Dark Daily reported on in November.
And in Switzerland, an eight-hospital medical group in Lugano partnered with Swiss Post (Switzerland’s postal service) and transportation technology manufacturer Matternet of Menlo Park, Calif., to successfully use drones to transport medical laboratory samples between hospitals, as noted in another Dark Daily report.
As drone technology improves, use of UAVs to transport blood and medical supplies and clinical laboratory specimens from point A to point B will likely become commonplace. They soon could be overflying a neighborhood near you!
—Andrea Downing Peck
Related Information:
‘Uber for blood’: How Rwandan Delivery Robots Are Saving Lives
Tanzania Announces World’s Largest National Drone Delivery Network Partnering with Zipline
Tanzania on Way to Becoming Drone Champion
Zipline Expands its Medical Delivery Drones Across East Africa
Johns Hopkins Test Drones Travels 161 Miles to Set Record for Delivery Distance of Clinical Laboratory Specimens
Drones Used to Deliver Clinical Laboratory Specimens in Switzerland
Mar 2, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
Liquid biopsy tests hold much promise. But inconsistencies in their findings provoke scrutiny and calls from researchers for further development before they can be considered reliable enough for diagnostic use
Many commercial developers of liquid biopsy tests tout the accuracy and benefits of their diagnostic technology. However, there are an equal number of medical laboratory experts who believe that this technology is not yet reliable enough for clinical use. Critics also point out that these tests are being offered as Laboratory Developed Tests (LDTs), which are internally developed and validated and have not undergone regulatory review.
Dark Daily has published several e-briefings on researchers who have sent the same patient samples to different genetic testing labs and received back materially different test results. Now, a new study by Johns Hopkins University concludes that liquid biopsy technology “must improve” before it should be relied upon for diagnostic and treatment decision making.
‘Certification for Medical Laboratories Must Improve’
Liquid Biopsy is the term for drawing whole blood and looking for cancer/tumor cells circulating in the blood stream. This is one factor in the imprecision of a liquid biopsy. Did the blood sample drawn actually have tumor cells? After all, only a limited number of tumor cells, if present, are in circulation.
Researchers at The James Buchanan Brady Urological Institute at Johns Hopkins School of Medicine know this and recently compared results of two liquid biopsy tests to determine which one would be more beneficial for patients. They published their findings in the December issue of JAMA Oncology.

Gonzalo Torga, MD (above left), and Kenneth J. Pienta, MD (above right), are the two Johns Hopkins Medicine doctors who conducted the recent study into the efficacy of liquid biopsy laboratory developed tests (LDTs) offered by different medical laboratory companies. They published their findings in JAMA Oncology. (Photos copyright: Johns Hopkins.)
To perform the study, researchers collected blood samples from 40 patients with metastatic prostate cancer and sent the same patient samples to two different Clinical Laboratory Improvement Amendments (CLIA) licensed College of American Pathologists (CAP) accredited laboratories. The labs then performed DNA next-generation sequencing on the samples following the directions of the two liquid biopsy manufacturers.
In reporting the DNA findings and results from the two medical laboratory companies, researchers discovered that the results completely matched in only three of the 40 patients! The Johns Hopkins researchers are concerned that patients could be prescribed certain cancer treatments based on which lab company’s liquid biopsy test their physician orders, instead of an accurate identification of the unique mutations in their tumors.
“Liquid biopsy is a promising technology, with an exceptional potential to impact our ability to treat patients, but it is a new technology that may need more time and experience to improve,” Gonzalo Torga, MD, Postdoctoral Fellow and Instructor at Johns Hopkins, and the lead author of the study, told Forbes. “We can’t tell from these studies which laboratory’s panel is better, but we can say that certification for these laboratories must improve.”
Unlocking New View of Tumors
Two commercial tests were used for the study:
Guardant360 from Guardant Health, Inc., uses digital sequencing to analyze genomic data points at the single molecular level. It examines 73 genes, including all National Comprehensive Cancer Network (NCCN) listed genes. The test searches for DNA fragments among billions of cells and digitally tags each fragment. This process unlocks a view of tumors that is not seen with tissue biopsies, which helps doctors prescribe the best treatment options for a particular patient.
“As a simple blood test, it provides physicians with a streamlined, cost-effective method to identify genomic alterations that can comprehensively influence a patient’s therapy response,” Helmy Eltoukhy, PhD, co-founder and Chief Executive Officer at Guardant Health, told MDBR.
“The only way of keeping ahead of those diseases and tracking those mutations has been through surgery, through doing a tissue biopsy and physically cutting a piece of the tumor out and sequencing it,” Eltoukhy noted in an interview with Xconomy. “What we’re able to do is essentially get the same, or sometimes better performance to tissue biopsy, but through two teaspoons of blood.”
According to the Guardant Health website, it takes just 14 days for a full report from Guardant360 to reach the ordering physician. In addition, the blood test provides samples with an adequate level of cell-free DNA to test 99.8% of the time and reduces errors and false positives found in standard sequencing methods by 1,000 times. It is common for samples used for tissue sequencing to have insufficient DNA for testing 20% to 40% of the time.
“We believe that conquering cancer is at its core a big data problem, and researchers have been data-starved,” explained Eltoukhy in VentureBeat. “Our launch of the world’s first commercial comprehensive liquid biopsy sparked a boom in cancer data acquisition. Every physician who orders one of our tests, and every patient whose tumor DNA we sequence, adds to this larger mission by improving our understanding of this complex disease.”
PlasmaSELECT-R64, manufactured by Personal Genome Diagnostics (PGDx), evaluates a targeted panel of 64 genes that have biological and functional relevance in making treatment decisions. PGDx announced the expanded version of its PlasmaSELECT assay in March of 2017.
“We are proud to launch the revolutionary PlasmaSELECT 64 expanded assay just six months after we introduced the most accurate, clinically actionable liquid biopsy tumor profiling assay to the market,” said Doug Ward, Chief Executive Officer at PGDx, in a press release. “This update is the first liquid biopsy assay that includes MSI (microsatellite instability) testing as a biomarker for high tumor mutational load, thereby providing cancer patients and their oncologists with information on whether they might be candidates for immuno-oncology therapies. The ability to generate DNA tumor profiling non-invasively using blood or plasma offers many advantages and makes genomic testing more accessible and usable.”
Regulations of LDTs Could be Needed to Improve Liquid Biopsy Tests
There are pathologists and clinical laboratory professionals who believe the technology behind liquid biopsies is not yet reliable enough for clinical use. The tests are being offered as LDTs, which are internally developed and validated, and the Food and Drug Administration (FDA) allows LDTs to be sold without regulatory reviews at this time. However, there are discussions regarding if and how to regulate LDTs, the outcome of which could impact how clinical laboratories are allowed to market the LDTs they develop.
Clearly, liquid biopsies are still in their relatively early stages of development. More testing and evaluation is needed to determine their efficacy. However, their potential to revolutionize cancer detection and care is obvious and a strong motivator for LTD developers, which means there will be future developments worth noting.
—JP Schlingman
Related Information:
Oncologists, Beware: Expensive Liquid Biopsy Tests Produce Conflicting Results
One Patient, Two Cancer DNA Tests, Two Different Results
Liquid Biopsy Results Differed Substantially Between Two Providers
Cancer Screening Firm Guardant Health Raises $360 Million to Sequence Tumor DNA of 1 Million Patients
Guardant Health Launches Guardant360 Blood Test in US
With $100M, Guardant Health to Expand Reach of Blood Test for Cancer
Personal Genome Diagnostics’ Expanded PlasmaSELECT 64 Is First Liquid Biopsy Pan-Cancer Profiling Panel to Include MSI Analyses for Immuno-Oncology
‘Liquid Biopsy’ Picks up Cancer Biomarkers in Blood, Study Finds
FDA Reveals New Approach to Laboratory Developed Tests
Using Extracellular Vesicles, Researchers Highlight Viability of Liquid Biopsies for Cancer Biomarker Detection in Clinical Laboratories
Feb 23, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
Researchers successfully isolated both plant and human RNA and DNA in the field, demonstrating the potential for their new dipstick technology to identify deadly bacteria, pathogens, and diseases in water, food, and even humans
Australian researchers at the University of Queensland (UQ) have developed an intriguing “dipstick” technology that might make it possible to use simple equipment to sequence DNA and RNA in the field. Among the potential applications that will interest clinical laboratory professionals is the ability for this technology to identify pathogens, both in humans and the environment.
Medical laboratories and anatomic pathologists are aware that gene sequencing (AKA, Nucleic Acid Sequencing) is the coming revolution in diagnostics. But the process is still costly and anchored to immovable technology that requires controlled environments and reliable resources. This promising new technology could make it simpler, cheaper, and faster to extract human DNA and RNA in settings outside a sophisticated core medical laboratory.
The UQ researchers developed technology that could affect how and where diagnostic tests for a whole range of pathogens are performed. For example, tests for bacteria such as E. coli in water supplies, pathogens in food, and diseases in humans currently are conducted in environmental and clinical laboratories. This new technology may allow such diagnostics to be done in extremely remote environments.
Isolating DNA/RNA in the Field
Jimmy Botella, PhD, Professor of Plant Biotechnology, and Michael Mason, PhD, Senior Post-doctoral researcher, both at the University of Queensland, led a team of researchers who published their findings in the journal PLOS Biology. The team developed a process they called “dipstick technology,” which allows DNA and RNA to be isolated quickly and without the use of specialized equipment.
They began by using the technology on particular plants, but soon found it could be used in many other situations.
“We found it had much broader implications as it could be used to purify DNA or RNA from human blood, viruses, fungi, and bacterial pathogens from infected plants or animals,” Botella noted in a press release.
The researchers’ objective was to investigate whether or not several different materials could be used to extract nucleic acids. “The first step in any application aiming to amplify DNA or RNA is the extraction of nucleic acids from a complex biological sample; a task traditionally requiring specialized equipment, trained technicians, and multiple liquid handling steps,” they wrote in the published study.

Holding the dipstick technology (from left) Dr. Michael Mason, Professor Jimmy Botella, and Yiping Zhou, all researchers at the University of Queensland in Brisbane, Australia. (Caption and photo copyright: University of Queensland.)
Their aim was to find a simpler process that required far less personnel and equipment. They found that cellulose-based filter paper could be used to bind nucleic acids. The filter paper, which was the control early in their investigation, even retained the nucleic acids through a purification process that removed contaminants. “We then adapted the cellulose filter to create a dipstick that can be used to purify nucleic acids from a wide range of plant, animal, and microbe samples in less than 30 seconds without the need for specialized equipment,” the researchers reported.
The team conducted its first tests on the plant species A. thaliana, a flowering plant found in Africa and Eurasia. However, wanting their dipstick technology to be useful in the field, they expanded their experiments to include various species of wheat, rice, soybean, tomato, and other plants. Citrus plants, known to be challenging, also were successfully tested.
The researchers then tested if their new technology would be useful for applications in humans, which is more complicated. HIV and hepatitis can be diagnosed using commercial kits, but those kits are not useful in many settings because the samples often require sophisticated manipulation. The researchers’ method—using cellulose paper and a one-minute wash—succeeded in amplification of the nucleic acid.
Performing Diagnostics in Hospitals, on Farms, and Even in the Jungle!
The University of Queensland’s commercialization company, UniQuest, has filed a patent application for the new technology. They are currently seeking partners to commercialize and sell the dipstick technology worldwide.
“Our dipsticks, combined with other technologies developed by our group, mean the entire diagnostic process from sample collection to final result could be easily performed in a hospital, farm, hotel room, or even a remote area such as a tropical jungle,” Botella noted in the press release.
The team conducted much of their field research on remote plantations in Papua New Guinea. They conducted tests on trees, livestock, human diseases, and to detect pathogens in food and water. “The dipstick technology makes diagnostics accessible to everyone,” Botella told Technology Networks.
Dipstick Diagnostics Not New to Point-of-Care Testing
As Modern Healthcare Executive noted, dipstick technology for various diagnostic purposes is not new, even though this particular application is, potentially, revolutionary. There are dipstick tests for everything from pregnancy to cholera. Also referred to as point-of-care testing (POCT), research and development of this technology has steadily grown, and as the UQ study shows, will likely continue.
In a paper published in Clinical Biochemistry Reviews, authors Andrew St. John, PhD, of ARC Consulting, and Christopher Price, MD, of the University of Oxford, noted, “Healthcare is changing, partly as a result of economic pressures, and also because of the general recognition that care needs to be less fragmented and more patient-centered.”
While there are certainly advantages to quick diagnostic tests that can be conducted in the field, there are some challenges, as well. Julie L. V. Shaw, PhD, Assistant Professor, Department of Pathology and Laboratory Medicine at The University of Ottawa, argues that “there are many challenges associated with POCT, mainly related to quality assurance,” in a paper she published in the journal Practical Laboratory Testing.
Technology will continue to develop and drive innovation and change in how diagnostics are performed and thus in how clinical laboratories operate. Various initiatives driving the industry toward personalized medicine and value-based care are sure to play a role, alongside new technology and other advancements.
With all of those changes, one thing remains critically important and that is the value of human understanding and innovation.
—Jillia Schlingman
Related Information:
Nucleic Acid Purification from Plants, Animals and Microbes in Under 30 Seconds
UQ Dipstick Technology Could Revolutionize Disease Diagnosis
Dipstick Technology Enables Rapid Diagnosis Anywhere
Existing and Emerging Technologies for Point-of-Care Testing
Practical Challenges Related to Point of Care Testing
The March of Technology Through the Clinical Laboratory and Beyond











