Oct 1, 2018 | Digital Pathology, Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
New advancements in mHealth, though encroaching on testing traditionally performed at clinical laboratories, offer opportunity to expand testing to remote locations
Mobile technology continues to impact clinical laboratories and anatomic pathology groups and is a major driver in precision medicine, as Dark Daily has reported. Most of the mobile-test development which incorporates smartphones as the testing device, however, has been for chemistry and immunoassay types of lab tests. Now, a new developer in Monmouth Junction, NJ, has created a Complete Blood Count (CBC) test that runs on devices attached to smartphones.
Such devices enable doctors to order test panels for patients in remote locations that also may lack resources, such as electricity.
The developer is Essenlix and it calls its new testing device iMOST (instant Mobile Self-Testing). According to the company’s website, which is mostly “Under Construction,” iMOST can provide “accurate blood and other healthcare testing in less than 60 seconds by a smartphone and matchbox-size-attachment, anywhere, anytime, and affordable to everyone.”
The company description on the Longitude Prize website states that Essenlix “uses multidisciplinary approaches to develop a new innovative platform of simple, fast, ultrasensitive, bio/chemical sensing and imaging for life science, diagnostics, and personal health.
The Longitude Prize competition was established to promote the invention of “an affordable, accurate, fast and easy-to-use test for bacterial infections that will allow health professionals worldwide to administer the right antibiotics at the right time,” the website states.

The Essenlix iMOST mobile-testing device (above) connects to a smartphone (shown right) and enables clinical laboratory technicians to run tests in remote locations from samples taken at time the test. Though still in trials, iMOST, and other similar devices, promise to expand testing to outside of traditional medical laboratory locations and further promote precision medicine. (Photos copyright: Lydia Ramsey/Business Insider.)
Essenlix’s iMOST mobile testing system consists of:
- a mobile application (app);
- the device attachment, which goes over the phone’s camera; and,
- a cartridge that holds a sample of blood.
So far, there have been two trials with a total of 92 participants, comparing traditional CBC testing with the Essenlix test. The results were within the FDA’s requirements for allowable error, prompting Chou to tell Business Insider, “Our error is clearly smaller than the FDA’s requirement, so the data is very, very good.”
Chou and his team are working toward FDA approval.
Other Testing Devices That Attached to Smartphones
Aydogan Ozcan, PhD, Professor of Electrical Engineering and Bioengineering at UCLA, and Mats Nilsson, PhD, Professor and Scientific Director of the Science for Life Laboratory at Stockholm University, have developed an attachment that they say can transform “a phone into a biomolecular analysis and diagnostics microscope,” according to The Pathologist. Dark Daily has published many e-briefings on Ozcan’s innovations over the years.
Their goal, the researchers said, was to create technology that can be used in low- and middle-income areas (LMICs), as well as in more advanced locations, such as Sweden. “I’ve been involved in other projects where we’ve looked at point-of-care diagnostic approaches,” he said, “and it seems to be very important that the devices [do not] rely on wired electricity or networks to serve not only LMICs, but also modern, developed environments. It’s often difficult to find an available power socket in Swedish hospitals.”
The molecular diagnostic tests that can be done with smartphone attachments—such as those developed by Ozcan and Nilsson—represent another way of using a smartphone in the healthcare arena, The Pathologist points out. Their invention combines the smartphone’s native camera, an app, optomechanical lasers, and an algorithm contained within the attachment to carry out fluorescence microscopy in the field.
Future of Mobile-Testing
An article appearing in the Financial Times describes some of the ways mobile technology is changing healthcare, including diagnostics that have traditionally been performed in the medical pathology laboratories.
“Doctors scan your body to look for irregularities, but they rely on pathologists in the lab to accurately diagnose any infection,” the article notes. “There, body fluids such as blood, urine, or spit are tested for lurking microbes or unexpected metabolites or chemicals wreaking havoc in your body. Now companies are miniaturizing these tests to create mobile pathology labs.”
Apple introduced the first iPhone in 2007. It’s doubtful anyone imagined the innovations in diagnostics and pathology that would soon follow. Thus, trying to predict what may be coming in coming decades—or even next year—would be futile. However, scientists and researchers themselves are indicating the direction development is headed.
Should Essenlix and other mobile-lab-test developers succeed in their efforts, it would represent yet another tectonic shift for medical pathology laboratories. Clinical laboratory managers and stakeholders should be ready, for the words of the ancient Greek philosopher Heraclitus have never been truer: “Change is the only constant in life.”
—Dava Stewart
Related Information:
Mobile Phone Microscopy
How Smartphones Are Transforming Healthcare
This Startup Wants to Make Blood Testing as Easy as Snapping a Photo with an iPhone
Is mHealth an Opportunity or Threat to Medical Laboratories and Pathology Groups?
New FDA Regulations of Clinical Decision-Support/Digital Health Applications and Medical Software Has Consequences for Medical Laboratories
UCLA Device Enables Diagnosis of Antimicrobial Resistance in Any Setting; Could Save Lives Lost to Antimicrobial Resistant Bacteria
Lab-on-a-Chip Diagnostics: When Will Clinical Laboratories See the Revolution?
Tiny, Simple-to-Use Lensless Microscope Might Soon Find a Place in Pathology
Sep 26, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
New metalens technology from MGH and SEAS researchers gives greater endoscopic optical imaging resolution and sample detail for anatomic pathologists performing diagnostics
Anatomic pathologists and clinical laboratories know that biopsy samples are necessary to diagnose many diseases. But, current endoscopic imaging techniques used by physicians sometimes fail to clearly visualize disease sites. Consequently, biopsies collected during these procedures may make it harder for pathologists and physicians to diagnose certain diseases and health conditions.
Now, a combined team of endoscopic imaging experts at Massachusetts General Hospital (MGH) and flat metalens developers at Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS) have developed “a new class of endoscopic imaging catheters—termed nano-optic endoscopes—that overcomes the limitations of current systems.”
That’s according to an article in Nature Photonics that reported on the research team’s study, published in Phys.org.
These nano-optics involved “flat metalenses” that promise to sharpen clarity and increase resolution of endoscopic imaging technology In turn, this contributes to more accurate pathology diagnostics and improve patient outcomes, while furthering the aims of precision medicine.
“Metalenses based on flat optics are a game changing new technology because the control of image distortions necessary for high resolution imaging is straightforward compared to conventional optics, which requires multiple complex shaped lenses,” Federico Capasso, PhD, Robert L. Wallace Professor of Applied Physics and Vinton Hayes Senior Research Fellow in Electrical Engineering at SEAS, and co-senior author of the study paper, told Nature Photonics. “I am confident that this will lead to a new class of optical systems and instruments with a broad range of applications in many areas of science and technology.”
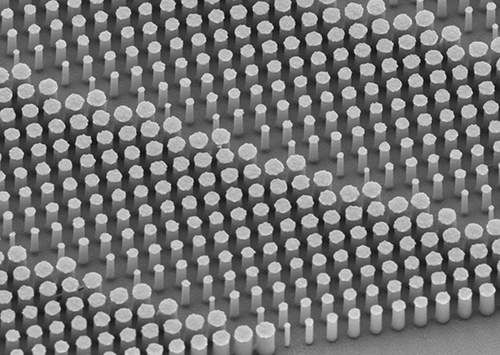
The image above shows a flat metalens taken using a scanning electron microscope. Anatomic pathologists and medical laboratories will benefit from the better quality biopsy specimens collected because of the sharper clarity and increased resolution of endoscopes built with the new nano-optics. (Photo copyright: Harvard SEAS.)
Researchers demonstrated the nano-optic endoscope’s ability to deeply penetrate and capture images at high resolutions in various tissues, including:
- Swine and sheep airways;
- Human lung tissue; and,
- Fruit flesh.
In the human lung tissue, “[T]he researchers were able to clearly identify structures that correspond to fine, irregular glands indicating the presence of adenocarcinoma, the most prominent type of lung cancer,” according to Phys.org.
Improving Endoscopic Imaging through Metalenses
The improved image resolution is due to the flat metalens configuration. “Currently, we are at the mercy of materials that we have no control over to design high-resolution lenses for imaging,” Yao-Wei Huang, PhD, Post-Doctoral Fellow at Harvard’s John A. Paulson School of Engineering and Applied Sciences and co-first author of the paper, told Phys.org.

Yao-Wei Huang, PhD (above), is a Post-Doctoral Fellow at Harvard’s John A. Paulson School of Engineering and Applied Sciences and co-first author of the study paper. “The main advantage of the metalens is that we can design and tailor its specifications to overcome spherical aberrations and astigmatism and achieve very fine focus of the light. As a result, we achieve very high resolution with extended depth of field without the need for complex optical components.” (Photo copyright: Harvard School of Engineering and Applied Sciences.)
The researchers note that current endoscopes using gradient-index (GRIN) lens-prism configurations and angle-polished ball lenses are used in a range of clinical applications due to their basic design. However, this benefit comes with shortfalls. “The ability of the nano-optic endoscope to obtain high-resolution images of sub-surface tissue structures in vivo is likely to increase the clinical utility of OCT [optical coherence tomography] in detection, diagnosis, and monitoring of diseases,” they state in their paper.
“The ability to control other properties of output light, such as the polarization state, enables a host of other applications—implausible to achieve using conventional catheters,” they continue. “Several tissues—such as smooth muscle, collagen (either innate or in fibrosis), and blood vessels—have constituent structures highly organized in one particular direction. Polarization-sensitive imaging can differentiate these structures from surrounding tissue by detecting their innate birefringence and optic axis.”
They further note that nano-optic endoscopes may yield benefits to other endoscopic optical imaging modalities such as confocal endomicroscopy.
Additional clinically-oriented studies will be required to assess how nano-optic endoscopes can elevate the capabilities of endoscopic OCT in examining fine pathological changes in luminal tissues.
Implications for Clinical Laboratories and Pathology Groups
The technology is still in the research stage with more trials needed to confirm the viability and accuracy of the approach. “This preclinical evaluation of the nano-optic endoscope indicated no significant flaws in the design for in vivo endoscopic imaging,” researchers note.
However, should nano-optic catheters gain clearance and change the endoscopy landscape as researchers predict, medical laboratories and pathologists might enjoy higher resolution images with greater information of the sample site—both key components of accurate diagnosis.
“Clinical adoption of many cutting-edge endoscopic microscopy modalities has been hampered due to the difficulty of designing miniature catheters that achieve the same image quality as bulky desktop microscopes,” Melissa Suter, PhD, Assistant Professor of Medicine at MGH and Harvard Medical School (HMS) and co-senior author of the study told Nature Photonics. “The use of nano-optic catheters that incorporate metalenses into their design will likely change the landscape of optical catheter design, resulting in a dramatic increase in the quality, resolution, and functionality of endoscopic microscopy. This will ultimately increase clinical utility by enabling more sophisticated assessment of cell and tissue microstructure in living patients.”
This research project at Massachusetts General Hospital and Harvard John A. Paulson School of Engineering and Applied Sciences is another example of how advances in technologies unrelated to surgical pathology can eventually contribute to improvements in how pathologists diagnose disease and help physicians identify the most promising therapies for their patients.
—Jon Stone
Related Information:
Nano-Optic Endoscope Sees Deep into Tissue at High Resolution
Nano-Optic Endoscope for High-Resolution Optical Coherence Tomography In Vivo
Nano-Optic Endoscope Allows High-Resolution Imaging
High-Resolution Nano-Optic Endoscope for Better Disease Detection
Sep 24, 2018 | Compliance, Legal, and Malpractice, Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations, News From Dark Daily
It’s the next wave in the long-running trend of hospital laboratory consolidation, as the need to trim costs and support thriving medical laboratory outreach programs continues
There’s an important new development in the hospital/health system sector of the clinical laboratory industry that continues the longstanding trend of consolidating multi-site lab operations. It is to rationalize and standardize medical laboratory operations across all lab sites within the health system. Effectively, this standardization trend represents the next cycle of clinical laboratory consolidation.
One recent example of this trend can be found at Atrium Health, the hospital health network based in Charlotte, N.C. (formerly known as Carolinas HealthCare System until earlier this year). Becker’s Hospital Review states that Atrium Health is the “seventh largest nonprofit system in the country based on number of acute-care hospitals (35).”
Creating Standardized Medical Laboratory Testing Services at Multiple Sites
Over the past four years, the clinical laboratory team at Atrium Health has worked to design, build, and operate a new, state-of-the-art core laboratory. At the same time, there were sequential projects to integrate the lab testing services and operations of nine other medical lab sites within the health system to better align the test menu, lab instruments, and workflow at these sites with the activities of the core laboratory.
According to Modena Henderson, MHA, the Vice President of Laboratory Services at Atrium Health, in an interview with Dark Daily, there were multiple primary goals in this project to rationalize and standardize lab testing at all the participating lab sites. They include:
- Standardizing lab test methodologies, reference ranges, and test menu;
- Standardizing analyzers and test platforms across all labs;
- Using Lean, Six Sigma, and other process improvement methods to streamline workflow and reduce test turnaround time;
- Improve productivity of lab staff;
- Increase quality while reducing or eliminating unproductive activities;
- Using real-time analytics middleware to keep lab management informed on a daily basis, and,
- Collaborating with emergency departments, wards, and outreach physicians to deliver more value with clinical lab testing services.
Using the ‘Three Ps of Project Management’ Approach in Health System Labs
The centerpiece of this program of lab rationalization and consolidation was the design and build-out for a new core clinical laboratory facility. Henderson said her team followed the principals of the “Three Ps of Project Management”—People, Process, Performance—to model the new lab facility, then guide how it was constructed and brought into daily clinical service.
“The Atrium Health laboratory regionalization project is an example of the next step that many innovative hospital laboratories are taking,” stated Robert L. Michel, Editor-in-Chief of The Dark Report. “Every lab has the same double challenge. First is financial. Hospital lab budgets are shrinking as growth in inpatient admissions slows. Outreach revenues are declining as Medicare and private payers slash lab test prices.
“Second, labs must come up with the capital needed to acquire and deploy the expensive and sophisticated new genetic and molecular tests that physicians and patients want,” he continued. “Hospital and health network labs must offer these new tests to keep their parent organizations at the cutting edge of clinical care.
Clinical Labs See Value in Standardizing Test Methodologies, Menus
“Thus, it is logical for the clinical labs of health networks to begin the process of rationalizing and standardizing their test menus, methodologies, and analyzers at every site within the system that performs medical lab testing,” emphasized Michel. “This is a development that we have watched gather momentum.”

Keynote Speaker Robert L. Michel, Editor-in-Chief of The Dark Report and Dark Daily will discuss how clinical laboratories of hospitals and health networks are rationalizing and standardizing their medical laboratory testing services to achieve the goals of managing lab costs, boosting quality, and increasing lab outreach revenue. The 12th annual Lab Quality Confab takes place on Oct. 9-10, 2018, at the Hyatt Regency Atlanta. (Photo copyright: The Dark Report.)
Michel offered two examples of sizable programs to rationalize and standardize clinical lab tests and services across a large health system. One is in Michigan, at Ascension Health. The other is in the Canadian Province of Québec. Both are large and ambitious undertakings, both in the number of lab sites involved and the large geography served by these clinical laboratories.
Consolidation Project in Québec involves 123 Clinical Lab Facilities
Québec’s provincial health system wants to consolidate 123 clinical laboratories in the province into 11 groups (clusters) of labs. Each lab group, or cluster, will have a core lab and rapid response labs. Test menus and methodologies will be standardized throughout the province. In an interview with The Dark Report, Ralph Dadoun, PhD, Project Director for Optilab Québec, plans to accomplish the consolidation without adding costs.
In Michigan, Ascension’s clinical lab leadership is working to integrate and standardize the labs that are operated by seven system organizations. This includes 14 hospitals and 18 existing laboratories located throughout the entire State of Michigan. In an interview with The Dark Report, Carlton Burgess, MSM, Vice President of Laboratory Services at Ascension Health’s St. John Providence Clinical Pathology Laboratory in Grosse Pointe Woods, Mich., stated that the goal is to have all the labs in the state work together in a seamless, integrated fashion.
Regional Lab Integration at North Carolina’s Biggest Health System
“To achieve this, the labs will be linked in four regions—a process we describe as regional integration,” explained Burgess. “Each region has a core lab and rapid response labs and each region will be responsible for building lab volume through increased outreach testing. In addition to changing how labs serve each region, our statewide standardization project has three objectives:
- “Repatriate existing send-out lab testing back into Michigan;
- “Establish standard test menus for each facility; and,
- “Renew each lab’s focus on growing lab outreach business.
“Every lab administrator and pathologist working in hospital and health network laboratories should be tracking this new trend of regionalization and standardization of hospital labs,” observed Michel. “That’s because labs already moving down this path are setting new standards for the entire clinical laboratory industry. This goes beyond cost and productivity, because these labs are putting the systems in place that will allow them to deliver more value to physicians and thus be paid more for that value by private health insurers.”
Innovative Lab Leaders to Speak at Lab Quality Confab in Atlanta
Lab leaders from Ascension Health will be keynote speakers at the upcoming 12th Annual Lab Quality Confab that takes place on October 9-10, 2018, at the Hyatt Hotel in Atlanta. They will also conduct multiple learning sessions to share their successes and lessons learned in building a new core laboratory and using that as a foundation to rationalize and standardize test methods, reference ranges, menus, lab automation, and analyzers at every clinical lab facility in the Ascension Health system. Sessions by Ascension Health lab leaders include:
- Leveraging Lean to become a Best-in-Class Lab Performer: How We Built and Automated a New Core Lab while Integrating Lab Operations and Helping Staff Embrace a New Culture; Modena Henderson, Vice President, Laboratory Services, and, Steven Harris, Assistant Vice President, Atrium Health.
- Achieving Standardized, High-Performance Lab Testing Services at Multiple Hospitals Using Lean Methods and Effective Engagement with Lab Staff and Nurses; Gary Catarella, MBA, MT(ASCP), Assistant Vice President, Hospital Operations, Atrium Health.
- Lessons We’ve Learned in Our Step-by-Step Journey to Transform Lab Operations and Integrate Testing across All Sites: Engaging Staff, Sustaining Change, Working with Vendors and Consultants—Interactive Roundtable Discussion; Modena Henderson, Vice President, Laboratory Services; and, Steven Harris, Assistant Vice President, Atrium Health.
Using Lean, Six, Sigma, ISO 15189 in Clinical Laboratory Operations
Lab Quality Confab this year features 60 speakers and 40 presentations from lab administrators, pathologists, and other lab managers on their successes and innovations using Lean, Six Sigma, ISO 15189, and other process management methods. You can view the full agenda here (or copy and paste this URL into your web browser: https://www.labqualityconfab.com/agenda).
This year’s Lab Quality Confab is on track to be the largest in its 12-year history. Limited spaces are still available. To ensure your place, register today at: https://www.labqualityconfab.com/register (or copy and paste this URL into your web browser: https://www.labqualityconfab.com/register).
Also, you can bring your lab team and make this Lab Quality Confab a group learning opportunity. When you bring four or more from your organization, each can register for $695 for this two-day learning event. One benefit you’ll gain from bringing your team is that it will give them the knowledge, the tools, and the confidence to help your lab reduce costs without compromising quality, while supporting sustained revenue growth from your hospital lab’s successful outreach program.
—Michael McBride
Related Information:
Full Agenda and Other Details for 12th Annual Lab Quality Confab
To Register for 12th Annual Lab Quality Confab
10 Things to Know about Atrium Health, Formerly Carolinas HealthCare System
Québec’s Laboratory Consolidation Plan Aims to Save $13.5 Million: Optilab Québec to move 123 labs into 11 lab groups
Michigan’s Ascension to Standardize Labs Throughout the State: Goals Are Common Test Methods, Menus, Practices
Sep 5, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory Pathology, Laboratory Testing
While approaches differ between the three companies, heavy investment in EMR/EHR and other HIT solutions could signal significant changes ahead for a market currently dominated by only a few major developers
If healthcare big data is truly a disruptive force in healthcare’s transformation, then a big battle looms for control of that data. Some experts say that the companies now dominating the electronic health record (EHR) market will soon face tough competition from the world’s biggest tech companies.
Until recently, most clinical laboratories, anatomic pathology groups, hospitals, and other healthcare providers have depended on EHR systems from just a handful of health information technology (HIT) developers. But tech giants Google, Apple, and Microsoft have been filing hundreds of HIT related patents since 2013 and appear poised to compete on a large scale for a chunk of the EMR/EHR/HIT market, according to coverage in EHR Intelligence of Kalorama Information’s “EMR 2018: The Market for Electronic Medical Records” report.
How this will impact medical laboratories and pathology practices remains to be seen. Labs are sure to be influenced by coming events, since clinical laboratory test data represents the largest proportion of an individual patient’s permanent medical record. It’s important to note, though, that while most EHR/HIT developers have been motivated by federal incentives, Google (NASDAQ:GOOG), Apple (NASDAQ:AAPL), and Microsoft (NASDAQ:MSFT) are motivated by consumer demand, which increasingly dictates the direction of health technology development.
Thus, they may be better positioned to compete moving forward, as patients, physicians, and hospitals turn to precision medicine and value-based care for improved outcomes and increased revenues.

“The EMR efforts have moved hospitals from paper to digital records,” Bruce Carlson (above), Publisher of Kalorama Information, told HIT Infrastructure. “The next step is for tech giants to glean the data and improve upon that infrastructure. We’ll be talking about EHR in different ways in the next ten years.” (Photo copyright: Twitter.)
EMR/EHR Market Poised for Disruption
According EHR Intelligence, as of 2017, 97% of all US non-federal acute care hospitals and 84% of US hospitals had adopted an EHR system. Of these hospitals, more than half (50.5%) use products from just two developers—Cerner or Epic. That’s according to Health Data Management’s coverage of the KLAS report “US Hospital EMR Market Share 2017.”
However, recent interest in HIT and EHR systems by major Silicon Valley tech companies could lead to potential disruptions in the current state of the market. According to The New York Times, in the first 11 months of 2017, 10 of the largest US technology companies were involved in healthcare equity deals worth $2.7-billion. This marks a drastic increase over the 2012 figure of $277-million.
Though each company is approaching the market differently, Google, Microsoft, and Apple are all working on projects that could influence how both consumers and healthcare professionals interact with and utilize medical record data.
Of the three, Apple is the most consumer-centric with their Apple Health personal health record (PHR) integration into Apple iOS for iPhones and iPads. Microsoft, however, is working on developing analytics tools and storage solutions aimed at healthcare providers in general. And Google, through its parent company Alphabet, is focusing on data processing and storage.
Amazon also is working on its own HIT project which it calls 1492. While details are scant, HIT Infrastructure reports that the project is focused on interoperability among disparate EHR systems to improve sharing of protected health information (PHI) between providers, patients, and other healthcare providers, such as clinical labs and pathology groups. HIT Infrastructure also reported on rumors of Amazon branching into telemedicine using their Amazon Echo and Alexa platforms.
Security Concerns and Opportunities for Clinical Laboratories
According to Computerworld’s coverage of IDC research, by 2020, 25% of patients are expected to be taking part in ‘bring your own data” healthcare scenarios. Tech-savvy medical laboratories could find opportunities to interact directly with patients and encourage follow-through on test orders or follow-up on routine testing.
However, shifting protected health information to devices carried by consumers is not without risks.
“How do I know the data won’t make its way to some cloud somewhere to be shared, sold, etc.” Jack Gold, Principal Analyst with J. Gold Associates, told Computerworld. “And if I rely on an app to tell me what to do—say, take my meds—and it somehow gets hacked, can it make me sick, or worse?”
These are important questions and developments, which Dark Daily has covered in other recent e-briefings. (See, “Apple Updates Its Mobile Health Apps, While Microsoft Shifts Its Focus to Artificial Intelligence. Both Will Transform Healthcare, But Which Will Impact Clinical Laboratories the Most?” July 25, 2018.)
Nevertheless, with tech giants already developing products for the consumer market and healthcare provider industry, it’s a given consumers will soon gain greater access to their own healthcare information. Whether patients will ultimately embrace it, how they will use it, and how developers will interact with the data, is still undefined. But it’s coming and clinical laboratories should be prepared.
—Jon Stone
Related Information:
Apple to Launch Health Records App with HL7’s FHIR Specifications at 12 Hospitals
How Google, Microsoft, Apple Are Impacting EHR Use in Healthcare
Microsoft, Apple, Google Secure HIT Infrastructure Patents
How Big Tech Is Going after Your Health Care
Amazon Secret Healthcare IT Tech Team Focuses on EHRs, Alexa
Apple’s Health Record API Released to Third-Party Developers; Is It Safe?
Apple, Cerner and Microsoft Are Interested in Buying AthenaHealth: Here’s Why This CEO Says They Won’t
Apple Says iOS Health Records Has over 75 Backers, Uses Open Standards
Report: Health Systems Share Apple Health Records Feedback
Apple Is Officially in the EHR Business. Now What?
Why Apple’s Move on Medical Records Marks a Tectonic Shift
Slideshow Where the Top 8 EMRs Are Deployed
Apple Updates Its Mobile Health Apps, While Microsoft Shifts Its Focus to Artificial Intelligence. Both Will Transform Healthcare, but Which Will Impact Clinical Laboratories the Most?
Apple’s Update of Its Mobile Health App Consolidates Data from Multiple EHRs and Makes It Easier to Push Clinical Laboratory Data to Patients
Aug 24, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing
CDC reports more than 93-million US adults are obese, and health issues related to obesity include heart disease, stroke, type 2 diabetes, and cancers
In recent years, the role of the human microbiome in weight loss or weight gain has been studied by different research groups. There is keen interest in this subject because of the high rates of obesity, and diagnostic companies know that development of a clinical laboratory test that could assess how an individual’s microbiome affects his/her weight would be a high-demand test.
This is true of a study published this year in Mayo Clinic Proceedings. Researchers at Mayo Clinic looked at obese patients who were in an active lifestyle intervention program designed to help them lose weight. It was determined that gut microbiota can have a role in both hindering weight loss and supporting weight loss.
Gut Microbiota More Complicated than Previously Thought
The Mayo researchers determined “an increased abundance of Phascolarctobacterium was associated with [successful weight loss]. In contrast, an increased abundance of Dialister and of genes encoding gut microbial carbohydrate-active enzymes was associated with failure to [lose] body weight. A gut microbiota with increased capability for carbohydrate metabolism appears to be associated with decreased weight loss in overweight and obese patients undergoing a lifestyle intervention program.”
How do bacteria impede weight loss? Vandana Nehra, MD, Mayo Clinic Gastroenterologist and co-senior author of the study, explained in a news release.
“Gut bacteria have the capacity to break down complex food particles, which provides us with additional energy. And this is normally is good for us,” she says. “However, for some individuals trying to lose weight, this process may become a hindrance.”
Put another away: people who more effectively metabolized carbohydrates were the ones who struggled to drop the pounds, New Atlas pointed out.

Vandana Nehra, MD (left), and Purna Kashyap, MBBS (right), are Mayo Clinic Gastroenterologists and co-senior authors of the Mayo study. “While we need to replicate these findings in a bigger study, we now have an important direction to pursue in terms of potentially providing more individualized strategies for people who struggle with obesity,” Nehra noted in the news release. Thus, precision medicine therapy for obese individuals could be based on Mayo Clinic’s research. (Photo copyright: Mayo Clinic.)
Mayo Study Provides Clues to Microbiota Potential in Weight Loss
The Mayo researchers wanted to know how gut bacteria behave in people who are trying to lose weight.
They recruited 26 people, ranging in age from 18 to 65, from the Mayo Clinic Obesity Treatment Research Program. Fecal stool samples, for researchers’ analysis, were collected from participants at the start of the three-month study period and at the end. The definition of successful weight loss was at least 5% of body weight.
Researchers found the following, according Live Science:
- 2 lbs. lost, on average, among all participants;
- Nine people were successful, losing an average of 17.4 lbs.;
- 17 people did not meet the goal, losing on average just 3.3 lbs.; and,
- More gut bacterial genes that break down carbohydrates were found in stool samples of the unsuccessful weight loss group, as compared to the successful dieters.
The researchers concluded that “An increased abundance of microbial genes encoding carbohydrate-active enzyme pathways and a decreased abundance of Phascolarctobacterium in the gut microbiota of obese and overweight individuals are associated with failure to lose at least 5% weight following a 3-month comprehensive lifestyle intervention program.”
Purna Kashyap, MBBS, Mayo Clinic Gastroenterologist and co-senior author of the study, told Live Science, “The study suggests there is a need to take the microbiome into account in clinical studies (on weight loss), and it also provides an important direction to pursue in terms of providing individualized care in obesity.” The very basis of precision medicine.
Future Weight-Loss Plans Based on Patient’s Microbiota
The Mayo Clinic researchers acknowledged the small sample size and need for more studies with larger samples over a longer time period. They also noted in their paper that Dialister has been associated with oral infections, such as gingivitis, and its role in energy expenditure and metabolism is unclear.
Still, the study suggests that it may soon be possible to give people individualized weight loss plans based on their gut bacteria. Clinical laboratory professionals and pathologists will want to stay abreast of follow-up studies and replication of findings by other research teams. A future medical laboratory test to analyze patients’ microbiomes could help obese people worldwide as well as lab business volume.
—Donna Marie Pocius
Related Information:
Gut Microbial Carbohydrate Metabolism Hinders Weight Loss in Overweight Adults Undergoing Lifestyle Intervention with a Volumetric Diet
Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice
CDC: Adult Obesity Facts
Makeup of an Individual’s Gut Bacteria May Play Role in Weight Loss, Mayo Study Suggests
Struggle to Lose Weight? Your gut Bacteria May Be to Blame
Your Gut Bacteria May Make It Harder to Lose Weight
Diet Hit a Snag? Your Gut Bacteria May be Partly to Blame
Can’t Lose Weight? Your Gut Bacteria Could be to Blame, According to Study
Richness of Human Gut Microbiome Correlates with Metabolic Markers
Annual Medical Spending Attributable to Obesity: Payer- and Service-Specific Estimates
5 Ways Gut Bacteria Affect Your Health
Cornell Researchers Identify Gut Microbes That May Help Some People Remain Thin; Findings Could Result in Clinical Laboratory Tests to Analyze Microbiomes of Individuals
Clinical Laboratories Might Soon be Diagnosing Obesity and Guiding Therapies that Utilize Engineered Microbes
Aug 20, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
“On-a-chip” devices continue to advance and medical laboratories will be natural repositories for patient data as the technology continues to improve
Dark Daily has predicted that the future of clinical laboratory testing will include highly complex multi-analyte test panels. The biomarkers, however, could number in the hundreds or thousands. So, it’s interesting to see new research by a Massachusetts Institute of Technology (MIT) team currently developing a multi-biomarker organ test device for clinical purposes.
Motivated by the costly failure of animal testing efforts to develop drug safety and efficacy in humans, the MIT research engineers created a microfluidic platform technology they dubbed “physiome-on-a-chip,” or more colloquially, “body-on-a-chip.” Their goal is to identify drug reaction in different cell groups within the body (in vivo).
They acknowledged contributions of in vitro microphysiological systems (MPSs), AKA “organ-on-a-chip” (OOC) systems. They note, however, in their paper published in Scientific Reports, that more complex systems that interconnect and receive data from multiple MPSs are needed due to increasing limitations arising from drugs’ “lack of efficacy” rather than toxicity.
“Here we describe the development and implementation of multi-MPS platforms, AKA physiome-on-a-chip, supporting four-way, seven-way, and 10-way MPS interactions for several weeks,” the MIT engineers wrote.
Though MIT’s new technology needs further research and development time, as well as clinical trials, this type of chip design and its ability to scale is a positive development and progress toward Dark Daily’s prediction. Once finalized, it could be adopted in medical laboratories for many types of diagnostic testing purposes.
Researchers Motivated to Improve Drug Efficacy
According to an MIT news release, “MIT engineers have developed new technology that could be used to evaluate new drugs and detect possible side effects before the drugs are tested in humans. Using a microfluidic platform that connects engineered tissues from up to 10 organs, the researchers can accurately replicate human organ interactions for weeks at a time, allowing them to measure the effects of drugs on different parts of the body.”
The “body-on-a-chip” technology, MIT says, is aimed at determining how drugs may affect one organ while also having side effects on others.
“Some of these effects are really hard to predict from animal models because the situations that lead to them are idiosyncratic. With our chip, you can distribute a drug and then look for the effects on other tissues and measure the exposure and how it is metabolized,” said Linda Griffith, PhD, Professor of Teaching Innovation at MIT’s School of Engineering, and a senior author of the study, in the news release.
According to MIT, factors affecting the effectiveness of pharmaceuticals may include:
- Genetics;
- Environment;
- Personal lifestyles; and,
- Interactions with other drugs.
TechCrunch called the study “unprecedented,” pointing to the platform’s connection of so many tissues and the technology’s ability to keep them stable for weeks.

“An advantage of our platform is that we can scale it up or down and accommodate a lot of different configurations,” Linda Griffith, PhD, MIT Professor, MIT School of Engineering, told Science Daily. “I think the field is going to go through a transition where we start to get more information out of a three-organ or four-organ system, and it will start to become cost-competitive because the information you’re getting is so much more valuable.” (Photo copyright: MacArthur Foundation.)
How “Body-on-a-Chip” Works
“Body-on-a-chip” is about the size of a tablet computer and links 10 organ types, including: liver, lung, gut, endometrium, brain, heart, pancreas, kidney, skin, and skeletal muscle.
Using microfluidic platform technology, the researchers placed one- to two-million cells from human tissue samples into the device and then pushed fluid through the chip to resemble blood flow, the Daily Mail reported, adding that MIT’s MPS platform design features:
- Compartments made from a plastic block;
- Passages for fluid to move (as a circulatory system does) between the compartments;
- A water reservoir to limit fluid evaporation; and,
- Ability to monitor flow of molecular exchanges and drug distribution.
Essentially, using the MIT device, a drug can be introduced to one organ, processed normally, and then passed to other organs for processing and use in other ways, TechCrunch summarized.
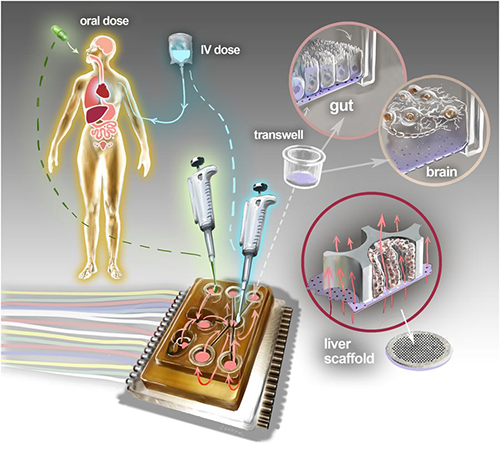
The physiome-on-a-chip system (above schematic) comprises bioengineered devices that nurture many interconnected 3D MPSs representing specified functional behaviors of each organ of interest, designed to capture essential features of in vivo physiology based on quantitative systems models tailored for individual applications such as drug fate or disease modeling. This technology could eventually be utilized for clinical laboratory and anatomic pathology testing. (Image and caption copyright: Victor O. Leshyk/Scientific Reports.)
Drug Delivery, Effects on Multiple Tissues Noted in MIT Study
The MIT researcher engineers reported these findings and accomplishments:
- Delivering a drug to the gastrointestinal tissue;
- Replicating digesting a drug;
- Observing as a drug was transported to other tissues and metabolized;
- Measuring a drug’s path; and,
- Noting effects of a drug on different tissues and how drugs break down.
“The huge potential of MPS technology is revealed by connecting multiple organ chips in an integrated system for in vitro pharmacology. This study beautifully illustrates that multi-MPS ‘physiome-on-a-chip’ approaches, which combine the genetic background of human cells with physiologically relevant tissue-to-media volumes, allow accurate prediction of drug pharmacokinetics and drug absorption, distribution, metabolism, and excretion,” said Kevin Healy, PhD, Professor of Bioengineering and Materials Science and Engineering, at University of California Berkeley in the MIT news release. Healy was not involved in the research.
Unique Device Design
In addition to making it possible to study so many different tissue types, the device design, according to MIT, is unique for these reasons:
- Its open microfluidic system, rather than a closed system, means the lid can be removed to manipulate tissue samples;
- Instead of external pumps common in closed systems, the MIT team used “on-board pumps” to control flow of liquid between the organs; and,
- The pumps used enabled larger engineered tissues, such as those from tumors in an organ, to be assessed.
The MIT engineers next plan to focus on specific organs—including the brain, liver, and gastrointestinal tissue—to model Parkinson’s disease, Digital Trends reported.
As healthcare providers and medical laboratories adopt precision medicine, MIT’s contributions are both timely and important. The ability to accommodate many different configurations in one platform is impressive, and something Dark Daily has been anticipating.
—Donna Marie Pocius
Related Information:
A “Body-on-a-Chip” Strings Together 10 Model Human Organs
“Body-on-a-Chip” Could Improve Drug Evaluation
MIT Builds “Body-on-a-Chip” Device That Can Store up to 10 Artificial Organs at Once
Interconnected Microphysiological Systems for Quantitative Biology and Pharmacology Studies
MIT Gadget Puts Multiple Artificial Organs into a Paperback-Sized Connected System
Drug Testing Could Get a Boost from MIT’s “Body-on-a-Chip”