Aug 10, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing
This low-cost, reusable noninvasive light test could serve as a prototype for detecting other biomarkers and diseases in rural and outlying medical laboratories
A 24-year-old Ugandan computer scientist whose own malaria was missed by traditional clinical laboratory blood tests has developed a device that detects signs of the disease using a beam of light directed onto a patient’s finger. The light highlights and detects changes in the color, shape, and concentration of red bloods cells affected by disease.
Brian Gitta, CEO and co-founder of computer software company thinkIT Limited, became the youngest winner of the UK’s Royal Academy of Engineering Africa Prize for Engineering Innovation. His eHealth solution is called Matibabu, which means “treatment” in Swahili.
Gitta and his team are developing a low-cost, reusable device that clips onto a patient’s finger and provides malaria test results within 60 seconds through a mobile phone app, UPI.com reported. The latest Matibabu prototype uses a ‘hybrid of magnetic-optic technology and electro-impedance technology’ to detect the disease,” according to a blog post on the thinkIT website.
“Our next step is to determine the validity and reliability of the Matibabu device compared with the gold standard microscopy and RDT by conducting field tests with malaria patients in selected health facilities in order to obtain information that will be used to improve the device, and eventually roll it out to the market,” the blog post states.

The Matibabu malaria detection device (above) uses the principles of light scattering and magnetism to detect changes to red blood cells that signal disease. The low-cost, reusable device from thinkIT Limited has advanced through several prototypes and now has an estimated 80% accuracy rate. (Photo copyright: Makerere University College of Engineering, Design, Art and Technology.)
TechCrunch reported that the current generation of Matibabu is about 80% accurate, with the expectation that further development will increase the device’s accuracy to 90-95%.
While this new diagnostic technology needs further development and clinical studies, it could potentially be used for other biomarkers and diseases besides malaria. However, according to the Centers for Disease Control and Prevention (CDC), rapid diagnostic tests (RDTs) like this are not yet widely used, so speed-of-diagnosis also is an issue.
Nevertheless, if successful, such a non-invasive test for malaria would be a major breakthrough since, today, the mosquito-borne disease must be confirmed by medical laboratory blood tests using either microscopic diagnosis or antigen detection, which are costly and time consuming.
“It’s a perfect example of how engineering can unlock development—in this case by improving healthcare,” Rebecca Enonchong, Africa Prize judge, noted in a Royal Academy of Engineering statement. “Matibabu is simply a gamechanger.”

Africa Prize judge Rebecca Enonchong (left) presents Ugandan Brian Gitta (right) of Matibabu with the Africa Prize winner’s medal. (Photo/caption copyright: Royal Academy of Engineering.)
Shafik Sekitto, Matibabu Vice President of Business Development, told BBC World News that Gitta’s own battle with malaria was prolonged because the first three blood tests failed to diagnose his disease. “[Gitta] brought up the idea: ‘Why can’t we find a new way of using the skills we have in computer science—of diagnosing a disease without having to prick somebody?’” Sekitto explained.
Malaria Threatens Half the World’s Population
The World Health Organization (WHO) estimates that nearly half the world’s population is at risk of malaria. According to WHO estimates, in 2015 there were 429,000 deaths worldwide from malaria, with 90% of cases and 92% of deaths in sub-Saharan Africa.
The Africa Prize, which includes a $33,400 (124-million Uganda shillings) award, is Africa’s biggest prize dedicated to engineering innovation. Its sponsors aim to encourage engineers from sub-Saharan Africa “to apply their skills to develop scalable solutions to local challenges.” In addition to funding, award recipients also receive business training, mentoring, and access to the Royal Academy of Engineering’s network of high profile and experienced engineers and experts, and their networks.
Gitta expects the award of the Africa Prize will help thinkIT Limited better navigate the difficult process of gaining worldwide regulatory approval for a new diagnostic device.
“It’s such a big achievement for us, because it means that we can better manage production in order to scale clinical trials and prove ourselves to regulators,” he predicted in the Royal Academy of Engineering statement. “The recognition will help us open up partnership opportunities—which is what we need most at the moment.”
Many pathologists and clinical laboratory managers are watching the efforts of various companies to develop medical laboratory tests that can be performed with a device that is coupled to a smartphone and can be performed as a point-of-care test. A substantial proportion of these research efforts are targeting the needs for accurate diagnostic testing in developing countries. That’s because of the need for cheap, fast, and accurate assays that can be performed in the rural areas of these nations.
—Andrea Downing Peck
Related Information:
Ugandan Inventor Wins Africa Prize for Bloodless Malaria Test
Ugandan Innovation Wins the Africa Prize for Engineering Innovation
Ugandan Wins Africa Prize for Bloodless Malaria Test
Matibabu Uses Light to Diagnose Malaria
Matibabu Wins the Africa Prize for Engineering Innovation
Aug 6, 2018 | Digital Pathology, Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing
Researchers in Boston are working to develop DNA as a low-cost, effective way to store data; could lead to new molecular technology industries outside of healthcare
Even as new insights about the role of DNA in various human diseases and health conditions continue to tumble out of research labs, a potential new use for DNA is emerging. A research team in Boston is exploring how to use DNA as a low-cost, reliable way to store and retrieve data.
This has implications for the nation’s clinical laboratories and anatomic pathology groups, because they are gaining experience in sequencing DNA, then storing that data for analysis and use in clinical care settings. If a way to use DNA as a data storage methodology was to become reality, it can be expected that medical laboratories will have the skillsets, experience, and information technology infrastructure already in place to offer a DNA-based data storage service. This would be particularly true for patient data and healthcare data.
Finding a way to reduce the cost of data storage is a primary reason why scientists are looking at ways that DNA could be used as a data storage technology. These scientists and technology developers seek ways to alleviate the world’s over-crowded hard drives, cloud servers, and databases. They hope this can be done by developing technologies that store digital information in artificially-made versions of DNA molecules.
The research so far suggests DNA data storage could be used to store data more effectively than existing data storage solutions. If this proves true, DNA-based data storage technologies could play a key role in industries outside of healthcare.
If so, practical knowledge of DNA handling and storage would be critical to these companies’ success. In turn, this could present unique opportunities for medical laboratory professionals.
DNA Data Storage: Durable but Costly
Besides enormous capacity, DNA-based data storage technology offers durability and long shelf life in a compact footprint, compared to other data storage mediums.
“DNA has an information-storage density several orders of magnitude higher than any other known storage technology,” Victor Zhirnov, PhD, Chief Scientist and Director, Semiconductor Research Corporation, told Wired.
However, projected costs are quite high, due to the cost of writing the information into the DNA. However, Catalog Technologies Inc. of Boston thinks it has a solution.
Rather than producing billions of unique bits of DNA, as Microsoft did while developing its own DNA data storage solution, Catalog’s approach is to “cheaply generate large quantities of just a few different DNA molecules, none longer than 30 base pairs. Then [use] billions of enzymatic reactions to encode information into the recombination patterns of those prefab bits of DNA. Instead of mapping one bit to one base pair, bits are arranged in multidimensional matrices, and sets of molecules represent their locations in each matrix.”
The Boston-based company plans to launch an industrial-scale DNA data storage service using a machine that can daily write a terabyte of data by leveraging 500-trillion DNA molecules, according to Wired. Potential customers include the entertainment industry, federal government, and information technology developers.
Catalog is supported by $9 million from investors. However, it is not the only company working on this. Microsoft and other companies are reportedly working on DNA storage projects as well.

“It’s a new generation of information storage technology that’s got a million times the information density, compared to flash storage. You can shrink down entire data centers into shoeboxes of DNA,” Catalog’s CEO, Hyunjun Park, PhD (above center, between Chief Science Officer Devin Leake on left and Milena Lazova, scientist, on right), told the Boston Globe. (Photo copyright: Catalog.)
Microsoft, University of Washington’s Synthetic DNA Data Storage
Microsoft and researchers at the University of Washington (UW) made progress on their development of a DNA-based storage system for digital data, according to a news release. What makes their work unique, they say, is the large-scale storage of synthetic DNA (200 megabytes) along with the ability to the retrieve data as needed.
“Synthetic DNA is durable and can encode digital data with high density, making it an attractive medium for data storage. However, recovering stored data on a large-scale currently requires all the DNA in a pool to be sequenced, even if only a subset of the information needs to be extracted,” the researchers wrote in their paper published in Nature Biotechnology.
“Here, we encode and store 35 distinct files (over 200 megabytes of data ) in more than 13-million DNA oligonucleotides and show that we can recover each file individually and with no errors, using a random access approach,” the researchers explained.
“Our work reduces the effort, both in sequencing capacity and in processing, to completely recover information stored in DNA,” Sergey Yekhanin, PhD, Microsoft Senior Researcher, told Digital Trends.
Successful research by Catalog, Microsoft, and others may soon lead to the launch of marketable DNA data storage services. And medical laboratory professionals who already know the code—the life code that is—will likely find themselves more marketable as well!
—Donna Marie Pocius
Related Information:
The Rise of DNA Data Storage
The Next Big Thing in Data Storage is Actually Microscopic
Catalog Hauls in $9 Million to Make DNA-Based Data Storage Commercially Viable
UW and Microsoft Researchers Achieve Random Access in Large-Scale DNA Data Storage
Random Access in Large-Scale DNA Data Storage
Microsoft and University of Washington Show DNA Can Store Data in Practical Way
Aug 1, 2018 | Digital Pathology, Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
Identifying patients who will likely develop prolonged concussion symptoms could lead to new clinical laboratory tests and personalized medicine treatments
Researchers are homing in on a new diagnostic assay for concussion that could potentially generate significant numbers of test referrals to the nation’s clinical laboratories. This innovative work is targeting how concussions are diagnosed and treated.
Each year, thousands of children receive sports-related injuries, including concussions. There are ways for anatomic pathologists and hospital medical laboratories to diagnose concussions; however, testing can be invasive and doesn’t always reveal a complete picture of the injury state.
Additionally, about one third of children with concussions develop prolonged symptoms. However, when prescribing treatment plans, physicians have been unable to predict which patients are likely to recover quickly versus those who will have a longer recovery.
Now, researchers at Penn State College of Medicine (Penn State) believe they have discovered five microRNAs in saliva that could be used to identify patients who will likely experience prolonged concussion symptoms even one month after the initial injury.
The study also found that certain materials in saliva can help diagnose the severity of concussions and could hold the key to more effective clinical laboratory tests and personalized medicine treatments.
The Penn State researchers published their study results in JAMA Pediatrics, a publication of the Journal of the American Medical Association (JAMA).
Concussion Leading Sports-related Brain Injury
There are approximately 3.8 million sports and recreation-related traumatic brain injuries in the United States each year and the majority of those cases are concussions, according to The Concussion Place. Most concussions treated in emergency rooms are due to falls, motor-vehicle related injuries, being struck by an object, assaults, or playing sports.
Also known as mild traumatic brain injuries (mTBI), concussions are caused by blows or jolts to the head or body that cause the brain to move with excessive force inside the skull. The sudden impact damages brain cells and causes chemical changes within the brain that alter normal functioning. Though usually not life threatening, the damage can be serious and linger for months.
Symptoms of concussion include: headaches, fatigue, nausea, vomiting, dizziness, balance problems, confusion, memory problems, sleep disturbances, and double or blurry vision. Symptoms usually occur immediately, but could take days or even weeks to appear.
Identifying Severity/Predicting Prolonged Symptoms of Traumatic Brain Injuries
After a concussion occurs, brain cells release small fragments of genetic material known as microRNAs while they attempt to repair themselves. A portion of these microRNAs appear in the injured person’s blood and saliva.
In order to determine whether these microRNAs could be used to determine the severity of a traumatic brain injury and predict whether prolonged symptoms would occur, the prospective cohort study researchers gathered saliva samples from 52 concussion patients between the ages of seven and 21:
- The average age of the subjects was 14;
- Twenty-two of the participants were female;
- They were all athletes; and,
- The majority of the samples were collected one to two weeks after the initial injury.
The researchers examined distinct microRNAs in the samples and identified some that enabled them to predict how long a patient’s concussion symptoms might last. In addition, they found one microRNA in children and young adults that accurately predicted which subjects would experience memory and problem-solving difficulties as part of their symptomatology.
The researchers also evaluated the concussion patients using the Sport Concussion Assessment Tool (SCAT-3), Third Edition. Physicians use this questionnaire to assess the symptoms and severity of concussions. The researchers also asked the parents of the concussed patients for observations about their children’s symptoms.
During follow up visits, which occurred at four- and eight-week increments following the original assessment, the Penn State researchers collected additional saliva samples and re-evaluated the patients using SCAT-3.
New Biomarkers Based on MicroRNAs Instead of Protein
“There’s been a big push recently to find more objective markers that a concussion has occurred, instead of relying simply on patient surveys,” Steven Hicks, MD, PhD, Assistant Professor of Pediatrics, Penn State College of Medicine, Hershey, Pa., one of the study researchers, told Penn State News.
“Previous research has focused on proteins, but this approach is limited because proteins have a hard time crossing the blood-brain barrier. What’s novel about this study is we looked at microRNAs instead of proteins, and we decided to look in saliva rather than blood,” he noted.

According to Steven Hicks, MD, PhD (above), who worked on the Penn State College of Medicine study, microRNAs could be more accurate than the traditional questionnaire when diagnosing and forecasting the effects of concussions. “The microRNAs were able to predict whether symptoms would last beyond four weeks with about 85% accuracy,” he told Penn State News. “In comparison, using the SCAT-3 report of symptoms alone is about 64% accurate. If you just go off the parent’s report of symptoms, it goes down to the mid-50s. In this pilot study, these molecular signatures are outperforming survey tools.” (Photo copyright: MD Magazine.)
The goal of this research was to develop a way to definitively ascertain that a concussion had occurred, predict the length and type of symptoms, and then use that data to improve and personalize care for children and young adults who have had a concussion.
“With that knowledge physicians could make more informed decisions about how long to hold a child out of sports, whether starting more aggressive medication regimens might be warranted, or whether involving a concussion specialist might be appropriate,” Hicks told MD Magazine. “Anytime we can use accurate, objective measures to guide medical care, I think that represents an opportunity to improve concussion treatment.”
Further research and clinical trials will be needed to solidify the effectiveness and accuracy of these new biomarkers. However, a rapid, non-invasive saliva test that can determine the severity of a concussion, and predicted whether prolonged symptoms will likely occur, would be widely used and could be an important assay for clinical laboratories. Particularly those associated with hospital medical laboratories and emergency rooms.
—JP Schlingman
Related Information:
Association of Salivary MicroRNA Changes with Prolonged Concussion Symptoms
Saliva Test May Detect Biomarker for Prolonged Concussion
Molecules in Spit May be Able to Diagnose and Predict Length of Concussions
Prolonged Concussion Symptoms Identifiable by Salivary MicroRNA
Spit Test May Help Reveal Concussion Severity
Saliva Test May Lead to Improved Concussion Care for Youths
Jul 27, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Pathology, Management & Operations, News From Dark Daily
Tuft’s proof-of-concept demonstration study shows how changes in saliva can be employed as biomarkers for development of future diagnostic monitoring devices and applications
For years, pathologists and dentists have recognized that the mouth contains many useful biomarkers for a wide range of health conditions and diseases. Now a study by a research team at Tufts University School of Engineering (Tufts) has demonstrated that a tooth-mounted sensor can reliably measure certain target markers.
In this proof-of-concept study, Tufts researchers developed a tooth-mounted sensor that monitors food consumption as it enters the body. This potentially adds behavioral data to the growing list of exploitable biomarkers available to developers of in vitro diagnostics (IVDs) and wearable medical monitoring devices. For that reason, many clinical laboratory managers and anatomic pathologists will want to track further development of this technology, which uses the mouth as the source of the markers to be measured.
A report detailing the device was first published in the scientific journal Advanced Materials in March of this year.
Sensor Reacts to Biomarkers in Saliva
The 2×2-millimeter flexible sensor consists of three layers and adheres to the tooth like a sticker. It has two gold outer rings surrounding an inner layer of bio-responsive material that is highly sensitive to glucose, salt, and alcohol. The presence of any of these substances alters the electrical properties of the sensor and incites it to transmit radio frequency waves that can be received by mobile devices.
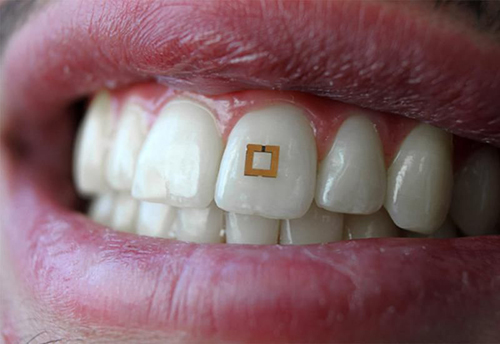
Researchers conducting a proof-of-concept study at Tufts University School of Engineering have developed “a materials‐based strategy to add utility to traditional dielectric sensors by developing a conformal radiofrequency (RF) construct composed of an active layer encapsulated between two reverse‐facing split ring resonators,” their paper published in Advanced Materials notes. The sensor is shown above mounted to a tooth, where it reacts to the presence of certain biomarkers in the saliva, triggering the transmission of an RFID signal. This device has the potential to also measure the same biomarkers used in clinical laboratory tests. (Photo copyright: Smithsonian Magazine/Tufts University School of Engineering.)
There are many possible uses for this tooth-mounted sensor. Individuals with medical conditions such as diabetes, celiac disease, or hypertension, which require them to avoid certain substances in their diet, could benefit from utilizing a device that employs the technology under development at Tufts.
Such a gadget might also help those trying to lose weight. The creators hope to enhance the material, so it has the ability to discern additional nutrients and chemicals.
“If you can evolve the sensor and engineer it to have a database of food consumption, then you could think about nutrition management,” Fiorenzo Omenetto, PhD, Professor, Department of Biomedical Engineering at Tufts and one of the authors of the research told Smithsonian Magazine. “That could be reminding us that we’re indulging too much in sugar or something like that.”
It also could potentially detect physiological or chemical changes taking place in the body by detecting certain bio-markers in the saliva.
“In theory we can modify the bio-responsive layer in these sensors to target other chemicals. We’re really limited only by our creativity,” Omenetto noted in a news release. “We have extended common RFID [radio frequency identification] technology to a sensor package that can dynamically read and transmit information on its environment, whether it is affixed to a tooth, to skin, or any other surface.”
Other Food Intake Devices
There have been previous attempts to develop wearable devices that monitors food intake. However, those gadgets usually required the use of mouth guards and head gear, which are too cumbersome for continuous everyday use. The minute size of the Tufts tooth-mounted device renders it more practical for consumers. And, since it can be mounted anywhere on a tooth—front or back—it can be made undetectable while being worn.
“This study is an interesting proof-of-concept demonstration that small, wireless biosensors can detect changes in saliva due to the presence of compounds such as salt, sugar, and alcohol,” Ben Almquist, PhD, a lecturer in the Department of Bioengineering at Imperial College London, told Smithsonian Magazine.
“For instance, for continuous monitoring of food intake, the sensors will need to be robust enough to withstand abrasion during chewing,” Almquist noted. “In addition, foods are complex mixtures of compounds including salts, sugars and proteins, and the relative amounts of each that enter into saliva will depend on factors such as the nature of the food [i.e., cooked versus fresh], the amount of chewing, and the time in the mouth before swallowing.”
The device currently remains in the prototype stage and more testing will be needed to determine its efficacy and durability. However, the emergence of such wearable devices for medical use suggests valuable opportunities for clinical laboratories.
Because data captured from the tooth-mounted device is transmitted wirelessly, clinical laboratories could potentially store and monitor the data, compare the collected data to other medical laboratory test results for the same patient, then communicate that information to clinicians, other caregivers, and even the patients. This would be a new way for clinical laboratories to provide innovative, value-added services to healthcare professionals and consumers.
—JP Schlingman
Related Information:
This Tiny Tooth Sensor Could Keep Track of the Food You Eat
Scientists Develop Tiny Tooth-mounted Sensors That Can Track What You Eat
A New Tooth-mounted Sensor Will Soon Help You Lose Weight
Functional, RF‐Trilayer Sensors for Tooth‐Mounted, Wireless Monitoring of the Oral Cavity and Food Consumption
Jul 25, 2018 | Digital Pathology, Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
While Apple recently debuted features to bring personal health records and protected health information to its mobile devices, Microsoft shuttered HealthVault in favor of focusing on AI-powered healthcare advances
As clinical laboratories and anatomic pathology groups know, lab testing data comprise more than 70% of the average patient’s health record. Thus, creating a universal platform on which consumers can share or review health information and medical histories with caregivers is a critical, yet elusive goal for most major tech companies, including tech giants Apple (Nasdaq:AAPL) and Microsoft (Nasdaq:MSFT).
Apple has big plans for patient health records and is working to bring protected health information (PHI) and healthcare advice to iPhones, iPads, and Apple Watch. Meanwhile, Microsoft is reducing its footprint in the mobile device healthcare market. Instead, it appears to be banking on its Artificial Intelligence (AI) platform. How these two diverging paths play out could have ramifications for the pathology and clinical laboratory industries.
HealthVault Insights versus AI versus Apple Health Mobile Apps
Launched in February 2017, Microsoft’s HealthVault Insights combined machine learning and AI with patients’ PHI and mobile activity tracking. The intent was to create an accessible, interactive platform for patients to monitor important health trends.
However, as of January 2018, Microsoft pulled the mobile app from Android, iOS, and Windows App stores. While summary information that draws on previously collected data is still available from the HealthVault website, new data and detailed insights are no longer available.
“We launched HealthVault Insights as a research project … with the goal of helping patients generate new insights about their health,” states Microsoft’s HealthVault Insights website. “Since then, we’ve learned a lot about how machine learning can be used to increase patient engagement and are now applying that knowledge to other projects.”
According to ZDNet, the closing of HealthVault Insights does not impact the Microsoft Health platform or the HealthVault patient-records system.
However, Microsoft’s shuttering of HealthVault Insights, and Google’s shuttering its Google Health platform in 2012, does seem to make Apple the last major tech company developing apps target at healthcare consumers designed to help them exchange private health information with caregivers through mobile devices. Dark Daily reported on Apple’s update earlier this year. (See, “Apple’s Update of Its Mobile Health App Consolidates Data from Multiple EHRs and Makes It Easier to Push Clinical Laboratory Data to Patients,” March 21, 2018.)
AI Will ‘Dramatically Transform Healthcare’
Shuttering HealthVault highlighted Microsoft’s shift away from consumer-facing health efforts and toward assisting medical laboratories, physicians, and research groups discover and implement treatments driving modern personalized medicine.
In a Microsoft blog post, Peter Lee, Corporate VP of Microsoft Healthcare, stated that Microsoft hopes its Healthcare NeXT platform will “dramatically transform healthcare, will deeply integrate Greenfield research and health technology product development, as well as establish a new model at Microsoft for strategic health industry partnerships.”
HealthVault Insights was one of several projects in Microsoft’s Healthcare NeXT initiative. Run by Microsoft’s AI and Research Group and partnering with major healthcare and research facilities across the country, other projects in the Healthcare NeXT initiative include:
Speaking with Business Insider, Lee noted that healthcare is becoming a “very large business” for Microsoft. “We don’t talk publicly about the dollars, but it’s large,” he concluded.
Microsoft’s EmpowerMD website states the eventual goal is to use the system to connect conversations with the growing trove of healthcare data available. “Our long-term vision is a learning system that incorporates data from longitudinal medical records, medical devices, genomics, population health, research papers, and more.”
AI a ‘Sleeping Giant for Healthcare’
“AI can be viewed as a sleeping giant for healthcare,” Eric Horvitz, PhD, Director of Microsoft Research Labs, told Nasdaq, when discussing Microsoft’s view of technology and healthcare. “AI methods show promise for multiple roles in healthcare. [This includes] inferring and alerting about hidden risks of potential adverse outcomes, selectively guiding attention, care, and interventional programs where [they are] most needed and reducing errors in hospitals.”
One such project involves a strategic partnership with the University of Pittsburg Medical Center (UPMC), which is a “$13-billion Pittsburgh-based system, comprising more than 25 hospitals, a three-million-member health plan, and 3,600 physicians, [that] will be a core partner in our efforts to improve healthcare delivery through a series of projects, beginning with a focus on transforming clinician empowerment and productivity,” according to Microsoft.

“Despite UPMC’s efforts to stay on the leading edge of technology, too often our clinicians and patients feel as though they’re serving the technology rather than the other way around. With Microsoft, we have a shared vision of empowering clinicians by reducing the burden of electronic paperwork and allowing the doctor to focus on the sacred doctor-patient relationship,” Steven D. Shapiro, MD (above), Chief Medical and Scientific Officer of UPMC and President of UPMC’s Health Services division, stated in the Microsoft blog. [Photo copyright: University of Pittsburg Medical Center.]
Medical laboratories and anatomic pathology groups may soon contribute health information to databases that one day will power AI systems. These trends highlight opportunities to both educate physicians on the tools available to utilize patient health data in an effective manner, and on new platforms that clinical laboratories could use to further streamline operations, reduce costs, and boost efficiency.
—Jon Stone
Related Information:
How Microsoft Is Using Advanced Technology in Healthcare
Microsoft Scrapping Personal Health Data App-Based Research Project
An Update on HealthVault Insights
How Microsoft’s Top Scientists Have Built a Big Business in Hacking Healthcare and Helped a Lot of People Along the Way
Microsoft Abandons Its Own HealthVault App: Is This Part of Something Larger?
Here’s How Microsoft Is Investing in AI
Microsoft Rolls Out More AI-Infused Healthcare Services, Software
Microsoft and Partners Combine the Cloud, AI, Research and Industry Expertise to Focus on Transforming Health Care
In Healthcare Push, Microsoft Launches Genomics Service on Azure Cloud
Apple’s Update of Its Mobile Health App Consolidates Data from Multiple EHRs and Makes It Easier to Push Clinical Laboratory Data to Patients
Jul 23, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing
Here is another example of how the diagnostic technologies used by anatomic pathology and clinical laboratories are evolving toward minute devices that deliver data wirelessly to electronic health records and healthcare databases for access by physicians and lab professionals
Anatomic pathologists and clinical laboratory managers keeping track of new implantable patient monitoring devices should find Abbott Laboratories’ (Abbott) recent technology release of great interest.
It’s an insertable cardiac monitor (ICM), also known as an implantable loop recorder, called Confirm Rx that, once implanted, can continuously monitor a patient’s heart rate and, using Bluetooth wireless technology, transmit collected data to physicians via a smartphone app. Abbott claims it is the “world’s first smartphone-compatible ICM.”
The laboratory’s website states, “The Confirm Rx ICM offers convenient, connected, and continuous monitoring for insight into your patients’ conditions and symptoms, including syncope, palpitations, and [atrial fibrillation] before or after ablation therapy and cryptogenic stroke—with fewer interruptions to their daily lives.”
Healthcare providers can implant Confirm Rx underneath the skin of a patient’s chest in just a few minutes using a simple insertion tool. The monitoring device is about the size of a small paper clip and has a two-year battery life. It provides physicians and their patients with an innovative way to identify and monitor abnormal heart rhythms or cardiac arrhythmias.
Transmitting ICM Data Using Smartphone App
Data collected by the Confirm Rx can be transmitted to a healthcare professional using the myMerlin for Confirm Rx smartphone app, developed by St. Jude Medical, which is now Abbott Laboratories (NYSE:ABT). No home-based transmitter, handheld activator, or additional equipment is required. However, individuals who use the Confirm Rx need to keep their smartphones within five feet of their bodies to ensure a continuous connection.
“Confirm Rx shows what we can do with cutting edge communication technology and the most advanced medical devices that provide new opportunities to improve patient care,” stated Avi Fischer, MD, Divisional VP Medical Affairs, and Medical Director for Cardiac Rhythm Management at Abbott, in a news release.
“By offering a device that uses Bluetooth wireless technology from the patient’s smartphone, we can help physicians easily and remotely diagnose potentially dangerous abnormal heart beats without requiring the patient to use a separate or cumbersome recording device,” Fischer concluded.
The myMerlin mobile app transmits its data to the Merlin.net Patient Care Network, where doctors and patients can simultaneously track symptoms, synchronize personal data, and view a transmission history.
“Prior to the evolution of this technology, the ability to monitor patients was really short-lived. But this allows the monitoring to continue and allows the patient to continue to live their lives unhindered,” noted Sean C. Beinart, MD, Cardiologist and Medical Director, Cardiovascular Research Washington Adventist Hospital, in an Abbott product release.

“Whenever you’re dealing with rhythm problems in the heart, being able to identify risk can sometimes mean the difference between life and death. The key is finding out what happened: What’s the cause of your symptoms?” Sean C. Beinart, MD (above), FACC, FHRS, a Cardiologist and Medical Director, Cardiovascular Research Washington Adventist Hospital, stated in an Abbott Laboratories video. Click on this link to watch the video. (Caption and video copyright: Abbott Laboratories.)
Peace of Mind through Technology
According to Abbott’s website, up to 7.1 million people in the United States are living with irregular heartbeats. The stroke risk for these individuals is five times higher than the risk for those without arrhythmias. When properly diagnosed, the condition is highly treatable.
“The stakes are very high,” Beinart stressed. “What the Confirm Rx does is it gives both me and my patient peace of mind that we’ve got them covered.”
Abbott obtained the Confirm Rx device when it acquired St. Jude Medical last year. The US Food and Drug Administration (FDA) approved the device in October of 2017. The first implantation of the Confirm Rx device took place in November.
The feedback received from users regarding the Confirm Rx has been positive and has helped increase Abbott’s electrophysiology sales by 16% during the last quarter of 2017, Fischer told CNBC.
“The types of patients have varied from younger to older, and [the devices] are being implanted for a variety of conditions,” Fischer stated. “Sometimes it’s to diagnose or to rule something out.”
As more implantable diagnostic monitoring/reporting devices are brought to the market, it is expected that some will be designed to measure some of the same biomarkers used in the assays performed by clinical laboratories. As that happens, clinical labs will have opportunities to serve medical facilities, physicians, and patients in new and more timely ways.
These implantable diagnostic devices may also give medical laboratories another way to be of value in the collection, storing, analysis, and reporting of the data obtained by the monitors, along with other lab tests doctors may order.
—JP Schlingman
Related Information:
Medicine Is Moving from Implantables to ‘Chipables’
Heart Monitor Links Patients to Doctors Using the One Thing They’re Unlikely to Forget Their Phones
Abbott Launches the First and Only Smartphone Compatible Insertable Cardiac Monitor in the U.S.
Heart Monitoring, Everywhere You Go
Confirm Rx Patient Brochure (PDF)










