May 7, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory Pathology, Laboratory Testing
Genomic analysis of pipes and sewers leading from the National Institutes of Health Clinical Care Center in Bethesda, Md., reveals the presence of carbapenem-resistant organisms; raises concern about the presence of multi-drug-resistant bacteria previously undetected in hospital settings
If hospitals and medical laboratories are battlegrounds, then microbiologists and clinical laboratory professionals are frontline soldiers in the ongoing fight against hospital-acquired infections (HAIs) and antibiotic resistance. These warriors, armed with advanced testing and diagnostic skills, bring expertise to antimicrobial stewardship programs that help block the spread of infectious disease. In this war, however, microbiologists and medical laboratory scientists (AKA, medical technologists) also often discover and identify new and potential strains of antibiotic resistance.
One such discovery involves a study published in mBio, a journal of the American Society for Microbiology (ASM), conducted by microbiologist Karen Frank, MD, PhD, D(AMBB), Chief of the Microbiology Service Department at the National Institutes of Health (NIH), and past-president of the Academy of Clinical Laboratory Physicians and Scientists (ACLPS). She and her colleagues identified a surprising source of carbapenem-resistant organisms—the plumbing, sewers, and wastewater beneath the National Institutes of Health Center (NIHCC) in Bethesda, Md. And they theorize similar “reservoirs” could exist beneath other healthcare centers as well.
Potential Source of Superbugs and Hospital-Acquired Infections
According to the mBio study, “Carbapenemase-producing organisms (CPOs) are a global concern because of the morbidity and mortality associated with these resistant Gram-negative bacteria. Horizontal plasmid transfer spreads the resistance mechanism to new bacteria, and understanding the plasmid ecology of the hospital environment can assist in the design of control strategies to prevent nosocomial infections.”

Karen Frank, MD, PhD (above), is Chief of the Microbiology Service Department at the National Institutes of Health and past-president of the Academy of Clinical Laboratory Physicians and Scientists. She suggests hospitals begin tracking the spread of the bacteria. “In the big picture, the concern is the spread of these resistant organisms worldwide, and some regions of the world are not tracking the spread of the hospital isolates.” (Photo copyright: National Institutes of Health.)
Frank’s team used Illumina’s MiSeq next-generation sequencer and single-molecule real-time (SMRT) sequencing paired with genome libraries, genomics viewers, and software to analyze the genomic DNA of more than 700 samples from the plumbing and sewers. They discovered a “potential environmental reservoir of mobile elements that may contribute to the spread of resistance genes, and increase the risk of antibiotic resistant ‘superbugs’ and difficult to treat hospital-acquired infections (HAIs).”
Genomic Sequencing Identifies Silent Threat Lurking in Sewers
Frank’s study was motivated by a 2011 outbreak of antibiotic-resistant Klebsiella pneumoniae bacteria that spread through the NIHCC via plumbing in ICU, ultimately resulting in the deaths of 11 patients. Although the hospital, like many others, had dedicated teams working to reduce environmental spread of infectious materials, overlooked sinks and pipes were eventually determined to be a disease vector.
In an NBC News report on Frank’s study, Amy Mathers, MD, Director of The Sink Lab at the University of Virginia, noted that sinks are often a locus of infection. In a study published in Applied and Environmental Microbiology, another journal of the ASM, Mathers noted that bacteria in drains form a difficult to clean biofilm that spreads to neighboring sinks through pipes. Mathers told NBC News that despite cleaning, “bacteria stayed adherent to the wall of the pipe” and even “splashed out” into the rooms with sink use.
During the 2011-2012 outbreak, David Henderson, MD, Deputy Director for Clinical Care at the NIHCC, told the LA Times of the increased need for surveillance, and predicted that clinical laboratory methods like genome sequencing “will become a critical tool for epidemiology in the future.”
Frank’s research fulfilled Henderson’s prediction and proved the importance of genomic sequencing and analysis in tracking new potential sources of infection. Frank’s team used the latest tools in genomic sequencing to identify and profile microbes found in locations ranging from internal plumbing and floor drains to sink traps and even external manhole covers outside the hospital proper. It is through that analysis that they identified the vast collection of CPOs thriving in hospital wastewater.
In an article, GenomeWeb quoted Frank’s study, noting that “Over two dozen carbapenemase gene-containing plasmids were identified in the samples considered” and CPOs turned up in nearly all 700 surveillance samples, including “all seven of the wastewater samples taken from the hospital’s intensive care unit pipes.” Although the hospital environment, including “high-touch surfaces,” remained free of similar CPOs, Frank’s team noted potential associations between patient and environmental isolates. GenomeWeb noted Frank’s findings that CPO levels were in “contrast to the low positivity rate in both the patient population and the patient-accessible environment” at NIHCC, but still held the potential for transmission to vulnerable patients.
Antibiotic-Resistance: A Global Concern
The Centers for Disease Control and Prevention (CDC) reports that more than two million illnesses and 23,000 deaths in the US are caused each year by antibiotic resistance, with 14,000 deaths alone linked to antibiotic resistance associated with Clostridium difficile infections (CDI). Worldwide those numbers are even higher.
Second only to CDI on the CDC’s categorized list of “18 drug-resistant threats to the United States” are carbapenem-resistant Enterobacteriaceae (CRE).
Since carbapenems are a “last resort” antibiotic for bacteria resistant to other antibiotics, the NIHCC “reservoir” of CPOs is a frightening discovery for physicians, clinical laboratory professionals, and the patients they serve.
The high CPO environment in NIHCC wastewater has the capability to spread resistance to bacteria even without the formal introduction of antibiotics. In an interview with Healthcare Finance News, Frank indicated that lateral gene transfer via plasmids was not only possible, but likely.
“The bacteria fight with each other and plasmids can carry genes that help them survive. As part of a complex bacterial community, they can transfer the plasmids carrying resistance genes to each other,” she noted. “That lateral gene transfer means bacteria can gain resistance, even without exposure to the antibiotics.”
The discovery of this new potential “reservoir” of CPOs may mean new focused genomic work for microbiologists and clinical laboratories. The knowledge gained by the discovery of CPOs in hospital waste water and sinks offers a new target for study and research that, as Frank concludes, will “benefit healthcare facilities worldwide” and “broaden our understanding of antimicrobial resistance genes in multi-drug resistant (MDR) bacteria in the environment and hospital settings.”
—Amanda Warren
Related Information:
Genomic Analysis of Hospital Plumbing Reveals Diverse Reservoir of Bacterial Plasmids Conferring Carbapenem Resistance
Snooping Around in Hospital Pipes, Scientists Find DNA That Fuels the Spread of Superbugs
CSI Bethesda: Sleuths Used Sequenced Genome to Track Down Killer
Antibiotic/Antimicrobial Resistance
Study Tracks How Superbugs Splash Out of Hospital Sink Drains
CDC: Biggest Threats
Antimicrobial Stewardship: How the Microbiology Laboratory Can Right the Ship
Superbugs Breeding in Hospital Plumbing Put Patients at Risk
Microbiologists at Weill Cornell Use Next-Generation Gene Sequencing to Map the Microbiome of New York City Subways
Apr 25, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Management & Operations
Direct-to-consumer medical laboratory testing company gets a major shot in the arm as developers find ready investors and increasing consumer demand
Clinical laboratory tests, usually performed without fanfare, were thrust into the limelight during a recent episode of Shark Tank, an American reality TV show on which aspiring entrepreneurs compete for the attention and partnership funds of various investors.
EverlyWell, a direct-to-consumer (DTC) company that offers at-home lab tests without lab visits or doctor referrals, obtained a $1-million line of credit from Lori Greiner, one of Shark Tank’s participating entrepreneurs, according to MobiHealthNews. EverlyWell has consumers collect their own specimens at home, which are then sent to a medical laboratory testing facility.
Based in Austin, Texas, EverlyWell was founded in 2015 by Julia Taylor Cheek, CEO, with an aim to “make lab tests accessible, simple, and meaningful,” according to a news release. Cheek is also a Venture Partner with NextGen Venture Partners and formerly the Director of Strategy and Operations with the George W. Bush Institute.
“It’s incredible for the industry that we were selected and aired on a show like Shark Tank. It really shows the intersection of what’s happening in consumer healthcare and the high cost in healthcare and that people are really responding to new solutions,” Cheek told MobiHealthNews.
“I think the product is brilliantly crafted,” Greiner stated during the episode’s taping, according to MobiHealthNews. “It’s really nice; it’s really easy. It’s super clear. I think the state of healthcare in our country now is so precarious. I think this gives people an empowered way … to know whether or not they have to go find a doctor,” she concluded.
Greiner offered the $1 million line of credit (with 8% interest) in exchange for a 5% equity stake in EverlyWell, explained Austin360. According to SiliconHillsNews, she did so after reviewing certain EverlyWell financial indicators, including:
- $2.5 million in revenue in 2016;
- $5 million expected revenue in 2017; and
- 20% monthly growth rate.

Julia Cheek, CEO and Founder of EverlyWell (above), in a news release following her success on reality show Shark Tank, said, “We’re leading a major shift in the consumer health marketplace by bringing the lab to consumers’ doorsteps, and we are moving quickly to expand our channels, launch innovative tests, and deliver a world-class customer experience.” (Photo copyright: Forbes/Whitney Martin.)
Physician Review Still Part of Home-testing Process
EverlyWell lists 22 home lab tests on its website and a market share that encompasses 46 states. Shoppers can search for specific tests based on symptoms or by test categories that include:
- General Wellness;
- Men’s Health;
- Women’s Health;
- Energy and Weight; and
- Genomic Test (through a partnership with Helix, a personal genomics company).
The most popular test panels include:
- Food sensitivity;
- Thyroid;
- Metabolism;
- Vitamin D; and,
- Inflammation.
Prices range from $59 for a glycated hemoglobin (HbA1c) test (found under the general wellness category) to $399 for a women’s health testing kit. EverlyWell explains that it has no insurance contracts for these diagnostic tests, which do not require office or lab visits.
The testing process, according to EverlyWell’s website, proceeds as follows:
- After ordering and paying online, kits arrive at the customer’s home;
- The consumer self-collects a sample (such as blood spots, dried urine, or saliva) and returns it by prepaid mail to a medical laboratory that partners with EverlyWell. The company notes that it works with CLIA (Clinical Laboratory Improvement Amendment)-certified laboratories;
- A board-certified doctor reviews the lab results; and,
- A report is available online in a few days.
“Our goal is not to remove the importance of physician review. It’s to make the experience easier for the consumer,” Cheek told Texas CEO Magazine. “We designed a platform that is all about access and empowering consumers to have access to and monitor their own health information,” she continued.
Texas CEO Magazine explained that Cheek was inspired to create the company following “a bad personal experience with health and wellness testing that sent her to seven different specialists, cost $2,000 out of pocket, and left her with pages of unreadable results.”
Since then, the three-year old start-up company has garnered more than $5 million in venture capital, noted the news release.
Many Choices in Direct-to-Consumer Lab Company Market
EverlyWell is not the only player in the DTC clinical laboratory test space. According to MedCityNews, there are at least 20 other DTC lab test companies in the market including:
- 23andMe;
- Laboratory Corporation of America (LabCorp);
- Mapmygenome;
- Pathway Genomics;
- Quest Diagnostics (Quest);
- Sonora Quest Labs;
- Theranos; and others.
The direct-to-consumer lab test market grew from $15 million to about $150 million in 2015 and includes both large and small clinical laboratory test developers, noted Kalorama Information.
Clearly, the DTC testing market is expanding and garnering the attention of major developers and investors alike. This growing demand for home-testing diagnostics could impact anatomic pathology groups and smaller clinical laboratories in the form of reduced order testing and decreased revenue.
—Donna Marie Pocius
Related Information:
Mail-Order Lab Test Startup EverlyWell Makes Million Dollar Deal on ABC’s Shark Tank
EverlyWell Raises Additional Capital, Bringing Total to $5 Million
This Austin Entrepreneur Scored Historic Deal on Shark Tank
Austin-based EverlyWell Lands Deal on Shark Tank
Innovative Texas Businesses: Empowering Consumers; Julia Cheek’s EverlyWell’s Health and Wellness Testing
Meet the Start-up Revolutionizing the Lab Testing Industry
20 Key Payers in the Direct-to-Consumer Lab Testing Market
Direct-to-Consumer Services Put Down Roots in US Lab Testing Market
Clinical Pathology Laboratories Should Expect More Direct-to-Consumer Testing
Sales of Direct-to-Consumer Clinical Laboratory Genetic Tests Soar, as Members of Congress Debate How Patient Data Should be Handled, Secured, and Kept Private
Apr 23, 2018 | Compliance, Legal, and Malpractice, Laboratory Hiring & Human Resources, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Management & Operations
Critics are quick to note that this creates a disparity in how patients access healthcare services
Independent concierge care (AKA concierge medicine) is available to anyone willing to pay the additional costs, which are over and above any health insurance. In a concierge care medical practice, patients pay an annual retainer fee to gain increased access to doctors, specialists, and services, such as faster TATs on clinical laboratory testing.
Depending on the program, concierge care also can offer patients a range of “improved” healthcare benefits, including same-day appointments, extended appointment times, around-the-clock telehealth services, and the experience of receiving care from a physician with a smaller patient roster and in a more personalized manner.
Clinical laboratories and anatomic pathology groups might also find benefit from the concierge care model. Though some concierge providers bill insurance, most work on a cash basis with payment due upfront for services. This ensures prompt payment for any medical laboratory testing provided, reduces administrative overhead, and eliminates the need to deal with payers.
Concierge Medicine Is Not Just for the Wealthy Anymore
Since its inception, concierge care has been considered a luxury available to only financially well-off patients. However, that may soon change. Several major health systems and hospitals are piloting scaled-back versions of concierge care aimed at both middle- and upper-class consumers. However, the programs are not without critics and have elicited both positive and negative responses from healthcare providers.
According to Modern Healthcare, hospitals and health systems currently testing concierge care programs include:
Patients with busy schedules or chronic conditions may see the biggest gains from investing in concierge care. The added flexibility and increased access might allow them to take advantage of care options more frequently. Physicians being able to take their time during consultations and more closely focus on specific concerns is also seen as a benefit to patients.
However, Modern Healthcare points out that patients are not the only ones to see benefits from this arrangement.
“Doctors who have switched to concierge-style medicine sing its praises, claiming the smaller patient panel allows the doctor to build relationships with patients and spend more time on preventive medicine,” Modern Healthcare noted.
In 2016, Dark Daily reported on similar findings from the American Academy of Private Physicians (AAPP). They noted that the average primary care physician in the US maintained between 2,000 and 4,000 patients using the traditional care model. In contrast, the AAPP found concierge physicians maintained on average only 600 patients. (See, “Concierge Medicine Increases in Popularity as More Consumers Opt for This Care Model; Will Clinical Laboratories Exploit This Business Opportunity?” May 6, 2016.)

Paul Huang, MD, PhD (above right), a concierge doctor at Massachusetts General Hospital, told Modern Healthcare, “We are not doing this just to make more money—we are doing this to make money to put back into the mission of the hospital and to support programs that otherwise would be difficult to support.” (Photo copyright: Modern Healthcare.)
Concierge Care: Controversial Approach or Major Boon to Hospitals?
Since its debut in the 1990s, concierge care has faced scrutiny and opposition from those who feel it discriminates against those who cannot afford retainer premiums and out-of-pocket expenses.
One health system that has drawn such criticism is Michigan Medicine (MM), which is owned by the University of Michigan. As reported by the Detroit Free Press, in a letter to hospital administration, 200 of MM’s own doctors and staff expressed their feelings about the concierge care program, stating, “Victors Care purports to offer ‘better’ healthcare to those with enough money to pay a large access fee. The University of Michigan is a public institution and our commitment is to serve the public, not a private few. We do not feel this is the role of a state university and are unable to justify this to the patients and families we serve.”
Tom Cassels, a consulting partner with the Advisory Board Company, told Modern Healthcare, “It’s a cultural learning curve, because most not-for-profit health systems are geared toward providing the same level of service to everyone in their community. The fundamental model of concierge medicine is to price-discriminate.”
However, media coverage also highlights how the hospitals creating concierge care services are using the financial benefits to help offset the cost of low-margin services or provide care to low-income patients who wouldn’t otherwise have access to care.
Misty Hathaway, Senior Director of the Center for Specialized Services at Mass General, explained to Modern Healthcare that since their physicians are salaried, margins from concierge services can help support “things like our substance abuse program, or other parts of primary care where the margin is a little bit harder to achieve.”
Despite the ethical debates, use of concierge care services continues to gain momentum as middle and upper-class patients find the increased quality of care a worthy value proposition. As more options emerge at major healthcare centers, medical laboratories and other service providers might find that this trend also offers an opportunity to increase revenue with a minimal impact on administrative and billing costs.
—Jon Stone
Related Information:
Concierge Care Taking Hold at Some Large, Urban Hospitals
No Appointment? No Problem … For a Price
Exclusive U-M Medical Plan Buys You ‘Better’ Care, Special Access
The Future of Healthcare Could Be in Concierge Medicine
The Doctor Won’t See You Now
Concierge Medicine Increases in Popularity as More Consumers Opt for This Care Model; Will Clinical Laboratories Exploit This Business Opportunity?
More Doctors Consider Concierge Medicine as Healthcare Reform Looms
Concierge Medicine Trend Continues and Creates New Clients for Clinical Pathology Laboratories
Apr 18, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
Three innovative technologies utilizing CRISPR-Cas13, Cas12a, and Cas9 demonstrate how CRISPR might be used for more than gene editing, while highlighting potential to develop new diagnostics for both the medical laboratory and point-of-care (POC) testing markets
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) is in the news again! The remarkable genetic-editing technology is at the core of several important developments in clinical laboratory and anatomic pathology diagnostics, which Dark Daily has covered in detail for years.
Now, scientists at three universities are investigating ways to expand CRISPR’s use. They are using CRISPR to develop new diagnostic tests, or to enhance the sensitivity of existing DNA tests.
One such advancement improves the sensitivity of SHERLOCK (Specific High Sensitivity Reporter unLOCKing), a CRISPR-based diagnostic tool developed by a team at MIT. The new development harnesses the DNA slicing traits of CRISPR to adapt it as a multifunctional tool capable of acting as a biosensor. This has resulted in a paper-strip test, much like a pregnancy test, that can that can “display test results for a single genetic signature,” according to MIT News.
Such a medical laboratory test would be highly useful during pandemics and in rural environments that lack critical resources, such as electricity and clean water.
One Hundred Times More Sensitive Medical Laboratory Tests!
Co-lead authors Jonathan Gootenberg, PhD Candidate, Harvard University and Broad Institute; and Omar Abudayyeh, PhD and MD student, MIT, published their findings in Science. They used CRISPR Cas13 and Cas12a to chop up RNA in a sample and RNA-guided DNA binding to target genetic sequences. Presence of targeted sequences is then indicated using a paper-based testing strip like those used in consumer pregnancy tests.
MIT News highlighted the high specificity and ease-of-use of their system in detecting Zika and Dengue viruses simultaneously. However, researchers stated that the system can target any genetic sequence. “With the original SHERLOCK, we were detecting a single molecule in a microliter, but now we can achieve 100-fold greater sensitivity … That’s especially important for applications like detecting cell-free tumor DNA in blood samples, where the concentration of your target might be extremely low,” noted Abudayyeh.

“The [CRISPR] technology demonstrates potential for many healthcare applications, including diagnosing infections in patients and detecting mutations that confer drug resistance or cause cancer,” stated senior author Feng Zhang, PhD. Zhang, shown above in the MIT lab named after him, is a Core Institute Member of the Broad Institute, Associate Professor in the departments of Brain and Cognitive Sciences and Biological Engineering at MIT, and a pioneer in the development of CRISPR gene-editing tools. (Photo copyright: MIT.)
Another unique use of CRISPR technology involved researchers David Liu, PhD, and Weixin Tang, PhD, of Harvard University and Howard Hughes Medical Institute (HHMI). Working in the Feng Zhang laboratory at the Broad Institute, they developed a sort of “data recorder” that records events as CRISPR-Cas9 is used to remove portions of a cell’s DNA.
They published the results of their development of CRISPR-mediated analog multi-event recording apparatus (CAMERA) systems, in Science. The story was also covered by STAT.
“The order of stimuli can be recorded through an overlapping guide RNA design and memories can be erased and re-recorded over multiple cycles,” the researchers noted. “CAMERA systems serve as ‘cell data recorders’ that write a history of endogenous or exogenous signaling events into permanent DNA sequence modifications in living cells.”
This creates a system much like the “black box” recorders in aircraft. However, using Cas9, data is recorded at the cellular level. “There are a lot of questions in cell biology where you’d like to know a cell’s history,” Liu told STAT.
While researchers acknowledge that any medical applications are in the far future, the technology holds the potential to capture and replay activity on the cellular level—a potentially powerful tool for oncologists, pathologists, and other medical specialists.
Using CRISPR to Detect Viruses and Infectious Diseases
Another recently developed technology—DNA Endonuclease Targeted CRISPR Trans Reporter (DETECTR)—shows even greater promise for utility to anatomic pathology groups and clinical laboratories.
Also recently debuted in Science, the DETECTR system is a product of Jennifer Doudna, PhD, and a team of researchers at the University of California Berkeley and HHMI. It uses CRISPR-Cas12a’s indiscriminate single-stranded DNA cleaving as a biosensor to detect different human papillomaviruses (HPVs). Once detected, it signals to indicate the presence of HPV in human cells.
Despite the current focus on HPVs, the researchers told Gizmodo they believe the same methods could identify other viral or bacterial infections, detect cancer biomarkers, and uncover chromosomal abnormalities.
Future Impact on Clinical Laboratories of CRISPR-based Diagnostics
Each of these new methods highlights the abilities of CRISPR both as a data generation tool and a biosensor. While still in the research phases, they offer yet another possibility of improving efficiency, targeting specific diseases and pathogens, and creating new assays and diagnostics to expand medical laboratory testing menus and power the precision medicine treatments of the future.
As CRISPR-based diagnostics mature, medical laboratory directors might find that new capabilities and assays featuring these technologies offer new avenues for remaining competitive and maintaining margins.
However, as SHERLOCK demonstrates, it also highlights the push for tests that produce results with high-specificity, but which do not require specialized medical laboratory training and expensive hardware to read. Similar approaches could power the next generation of POC tests, which certainly would affect the volume, and therefore the revenue, of independent clinical laboratories and hospital/health system core laboratories.
—Jon Stone
Related Information:
Multiplexed and Portable Nucleic Acid Detection Platform with Cas13, Cas12a, and Csm6
Rewritable Multi-Event Analog Recording in Bacterial and Mammalian Cells
CRISPR-Cas12a Target Binding Unleashes Indiscriminate Single-Stranded DNase Activity
Researchers Advance CRISPR-Based Tool for Diagnosing Disease
CRISPR Isn’t Just for Gene Editing Anymore
CRISPR’s Pioneers Find a Way to Use It as a Glowing Virus Detector
With New CRISPR Inventions, Its Pioneers Say, You Ain’t Seen Nothin’ Yet
New CRISPR Tools Can Detect Infections Like HPV, Dengue, and Zika
Breakthrough DNA Editing Tool May Help Pathologists Develop New Diagnostic Approaches to Identify and Treat the Underlying Causes of Diseases at the Genetic Level
CRISPR-Related Tool Set to Fundamentally Change Clinical Laboratory Diagnostics, Especially in Rural and Remote Locations
Harvard Researchers Demonstrate a New Method to Deliver Gene-editing Proteins into Cells: Possibly Creating a New Diagnostic Opportunity for Pathologists
Apr 2, 2018 | Digital Pathology, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing
Ever shrinking “lab-on-a-…” technologies, a boon to medical laboratories and anatomic pathologists in remote resource-strapped regions, also have a place in modern labs
Researchers took another leap forward in reducing the size of clinical laboratory diagnostic tests and observational tools. This demonstration involved lab-on-a-fiber technology and showed promise in both monitoring anatomic pathology biomarkers in vivo and supplementing the abilities of existing lab-on-a-chip and microfluidic devices.
Lab-on-a-Fiber Next Technological Step Toward Miniaturization
In 2013, Dark Daily reported on research into an implantable laboratory-on-a-chip (LOC) for monitoring blood chemistry during chemotherapy. It was a major breakthrough at the time, which promised new and powerful tools for cancer treatment regimens.
However, most LOC systems aren’t designed for wet environments. Also, while microfluidics and flexible membranes allow for smaller footprints and tighter placement, they are still invasive in ways that might make patients uncomfortable or make real-world use less than ideal. And, long-term use brings further complications, such as corrosion or foreign-body granulomas.
Thus, lab-on-a-fiber’s ability to function in vivo, is one of the device’s principal advantages, as ExtremeTech noted.
Lab-on-a-fiber technology addresses many concerns. It is small enough to insert directly into organs, muscle mass, or veins when used as biosensors. And the fibers can return a wealth of information by using light and reflection, while allowing for minimal discomfort and precision placement.
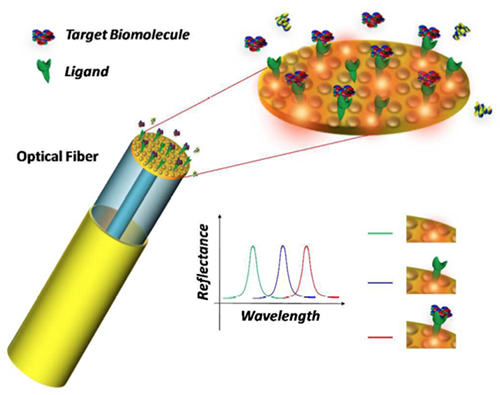
Schematic of the lab-on-a-fiber biosensing principle. A metallic nanostructure supporting a resonant plasmonic mode is integrated on the optical fiber tip. When a molecular binding event occurs at the sensor surface, the reflectance peak associated to the plasmonic mode shifts towards longer wavelengths. (Image and caption copyright: Analyst/The Royal Society of Chemistry.)
The Past and Future of Scaling Clinical Laboratory Testing
Dark Daily has followed these miniaturization trends for years starting with their earliest stages. A detailed timeline of developments can be viewed in “Lab-on-a-Chip Diagnostics: When Will Clinical Laboratories See the Revolution?” from 2016.
Additional Dark Daily “lab-on-a-…” coverage includes:
- IBM Watson Health and Mount Sinai Health System team up to use LOC solutions to separate biomolecules as small as 20nm from samples (See Dark Daily, “IBM and Mount Sinai Researchers Develop Innovative Medical Lab-on-a-Chip Solution,” October 3, 2016); and,
- Lab-on-Skin devices for monitoring biomarkers and electrophysiological signals, providing human-machine interfaces, and facilitating optogenetics (See Dark Daily, “In the Field of Nano-Scale Diagnostics, Many Researchers Are Developing ‘Lab-on-Skin’ Technologies That Can Monitor Many Clinical Laboratory Biomarkers,” January 15, 2015).
In the past year, a myriad of lab-on-a-fiber applications also have received media coverage, including:
Developers believe lab-on-a-fiber approaches could offer further adaptability and functionality to other “lab-on-a-…” technologies. For example, as highlighted in Advanced Science News, researchers are employing lab-on-a-fiber technologies to further refine and improve LOC functions and designs.
“As the scientific world moves inexorably to smaller dimensions … The emerging concept of ‘lab‐on‐fiber’ will give the optical fiber platform additional (highly integrated) functionalities,” noted Deepak Uttamchandani, PhD, Vice Dean Research, Faculty of Engineering, and, Robert Blue, PhD, Research Fellow, both at the University of Strathclyde, Glasgow, UK, in their review paper, “Recent Advances In Optical Fiber Devices for Microfluidics Integration.” The paper, published in the Journal of Biophotonics, examined “the recent emergence of miniaturized optical fiber-based sensing and actuating devices that have been successfully integrated into fluidic microchannels that are part of microfluidic and lab‐on‐chip systems.”

In his review paper on the emerging concept of lab-on-a-fiber, Deepak Uttamchandani, PhD, notes, “The versatility of the optical fiber platform has already allowed researchers to conduct immunoassays in microchannels using both fluorescently‐labelled and label‐free formats whilst gaining advantages of reduced assay time and increased sensitivity.” (Photo copyright: University of Strathclyde.)
Lab-on-a-Fiber: Another Step Forward or a Major Change?
At each milestone in the scaling of clinical laboratory testing, experts and media outlets predicted the demise of big laboratories and the dawn of a POC-centric testing era. Yet, despite 20-plus years of progress, this has yet to happen.
While it is critical for anatomical pathology leaders and clinical laboratory managers to stay abreast of developments in testing technology, much of the innovation behind lab-on-a-fiber remains strictly in the research realm. Challenges to the commercialization of these new techniques include both physical factors, such as design and manufacture of ready-to-use tests, and regulatory concerns, including FDA clearances and payer approval of new assays and diagnostic procedures.
Until researchers and test manufacturers overcome these hurdles, threats to current standards and workflows are minimal. However, much like the gains in scale realized through incorporating lab-on-a-chip concepts into clinical laboratory testing, the research powering these innovations might prove useful in further improving and expanding medical laboratory testing options.
—Jon Stone
Related Information:
Optical Fiber Devices for Microfluidics Integration Open Up New Horizons for Advanced “Lab-on-a-Chip” Technologies
Recent Advances in Optical Fiber Devices for Microfluidics Integration
Lab-on-Fiber Technology: A New Vision for Chemical and Biological Sensing [Abstract]
Lab-on-Fiber Technology: A New Vision for Chemical and Biological Sensing [Full Downloadable PDF]
How We’re Shrinking Chemical Labs onto Optical Fibers
Lab-on-Fiber Could Shine Light on Disease
Doctors Might Soon Diagnose You by Feeding a Lab-on-a-Fiber Straight into Your Veins
Fiber-Optic Device Can Detect Stray Cancer Cells and Improve Tumor Removal: Study
Fiber Optic Probe Beats a Biopsy for Measuring Muscle Health
Lab-on-a-Chip Diagnostics: When Will Clinical Laboratories See the Revolution?
Implantable Medical Laboratory-on-a-Chip Continuously Monitors Key Chemicals in Chemotherapy and High-Risk Patients
In the Field of Nano-Scale Diagnostics, Many Researchers Are Developing ‘Lab-on-Skin’ Technologies That Can Monitor Many Clinical Laboratory Biomarkers
Hematology on a Chip: University of Southampton Develops POC Blood Analysis
Sleek ‘Lab in a Needle’ Is an All-in-One Device That Detects Liver Toxicity in Minutes during a Study, Showing Potential to Supplant Some Medical Laboratory Tests
Whole Animal Assays Use Lab-on-a-Chip at MIT
IBM and Mount Sinai Researchers Develop Innovative Medical Lab-on-a-Chip Solution
In the Field of Nano-Scale Diagnostics, Many Researchers Are Developing ‘Lab-on-Skin’ Technologies That Can Monitor Many Clinical Laboratory Biomarkers
Mar 28, 2018 | Laboratory Hiring & Human Resources, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
Microhospitals already offer most of the critical features of traditional hospitals, and by featuring telemedicine technology at the point of care, they are becoming powerful tools for healthcare providers
Dark Daily reported in January that microhospitals are opening nationwide, including in such innovative states as Texas, Colorado, Nevada, and Arizona. In addition to being open 24/7 and mostly located in high-density areas, these scaled down hospitals feature the most critical aspects of full-size hospitals—medical laboratories, emergency departments, pharmacies, and imaging centers.
However, a report by the Health Resources and Service Administration (HRSA) predicted that by 2020 the US will be short as many as 20,000 primary care physicians! Many specialty practices also are expected to see stiff shortages of physicians in the near future. Without enough physicians, even microhospitals cannot provide adequate care.
Thus, the ever increasing practice of using telemedicine as a way to serve more people, while providing faster, more efficient care tailored to meet the needs of individuals and communities, is welcomed news. If this trend becomes more widespread, it will create new opportunities and challenges for clinical laboratories in hospitals, as well as health systems that own and operate microhospitals.
Filling a Need in Vulnerable Communities
At the end of 2016, there were approximately 50 microhospitals operating in the United States, mostly in the Midwest, Arizona, Colorado, Nevada, and Texas. Sometimes referred to as neighborhood or community hospitals, microhospitals are acute care facilities that are smaller than traditional hospitals but can deliver many of the same medical services. They provide more comprehensive treatments than typical urgent care and outpatient centers and fill a gap between freestanding healthcare centers and major hospitals.
Microhospitals typically have less than a dozen short-stay beds and have the ability to provide inpatient care, emergency care, and imaging and medical laboratory services. And, they are usually affiliated with larger healthcare systems, which allows them to expand into certain areas without incurring the high costs of building a full-scale hospital.
“Right now they seem to be popping up in large urban and suburban metro areas,” Priya Bathija, Vice President, Value Initiative American Hospital Association, told NPR. “We really think they have the potential to help in vulnerable communities that have a lack of access.”
Patient Satisfaction and Declining Physician Populations Drive Demand for Telemedicine
Telemedicine, a combination of telecommunications and information technology, is primarily used to remotely connect healthcare providers to patients during office visits. But it also is being used successfully at the point of care in emergency departments and even surgery.
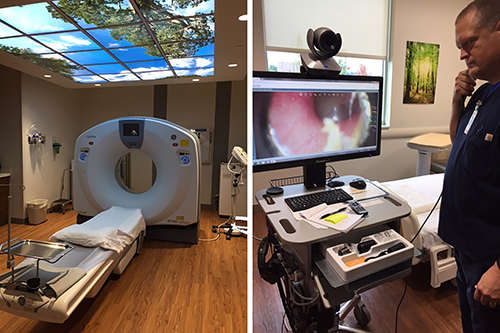
Microhospitals like St. Vincent Neighborhood hospital in Noblesville, Ind., which offer most of the critical functions of traditional hospitals, such as clinical laboratories, ERs, and the CT scanning station above (left), are increasingly including telemedicine technologies (above right) at the point of care to offset reductions in primary care and specialty physicians. (Photo copyright: Jill Sheridan/IPB News.)
Consumers are becoming more accepting of telemedicine (AKA, telehealth) as these services offer savings in both time and money. A recent survey by the Health Industry Distributors Association (HIDA) found that many patients were pleased with telehealth services. More than 50% of the surveyed individuals stated they were very satisfied with a recent telemedicine encounter. In addition, 54% of those individuals described their telehealth experience as better than a traditional, in-person office visit.
Telemedicine and Microhospitals Mutually Beneficial, According to HRSA
Other research suggests microhospitals may generate a mutually beneficial alliance with telemedicine that increases the progress of both entities, especially when considering projected increases in the number of nurse practitioners and physician assistants.
In its report, “Projecting the Supply and Demand for Primary Care Practitioners Through 2020,” Health Resources and Service Administration (HRSA) estimates there will be a shortage of more than 20,000 primary care physicians working in the US by the year 2020. Other specialties expected to experience staff shortfalls include:
Anticipation of this decline in physician numbers is fueling the demand for telemedicine to help with patient loads, especially in remote areas.
Saving Time and Money with Televisits
A study by Nemours Children’s Health System indicates that telemedicine may reduce medical costs for both patients and healthcare providers while sustaining patient satisfaction.
“At Nemours, we’ve seen how telemedicine can positively impact patients’ lives,” Shayan Vyas, MD, Medical Director of Telehealth at Nemours, noted in a press release. “The overwhelmingly positive response we’ve seen from parents who are early adopters of telemedicine really reinforces the feasibility of online doctor visits and sets the stage for real change in the way healthcare is delivered.”
The Nemours study involved 120 patients under the age of 18. The majority of families surveyed stated they would be interested in future telehealth visits and an impressive 99% said they would recommend telemedicine to other families.
The study discovered that patients and family members saved an average of $50 and about an hour of time, by utilizing telehealth for sports medicine appointments. The health system also experienced some monetary perks with the televisits, as they cost approximately $24 less per patient.
“We know that telemedicine is often looked to for common childhood ailments, like cold and flu, or skin rashes. But we wanted to look at how telemedicine could benefit patients within a particular specialty such as sports medicine,” Alfred Atanda Jr, MD, Pediatric Orthopedic Surgeon at Nemours/Alfred I. DuPont Hospital for Children in Wilmington, DE., told FierceHealthcare. “As the healthcare landscape continues to evolve and the emphasis on value and satisfaction continues to grow, telemedicine may be utilized by providers as a mechanism to keep costs and resource utilization low, and to comply with payer requirements.”
Healthcare consumers and providers are increasing looking to technology to enhance medical care and solve resource shortfalls. Separately, telehealth and microhospitals already help with these needs, Combined, however, they are a powerful solution to our nation’s reducing ranks of primary care physicians and medical specialists.
If this trend of microhospitals using telemedicine should continue and increase, both components will give medical professionals vital tools to provide faster, more economical, and more personalized services, to more patients across wider areas of America.
—JP Schlingman
Related Information:
Why Telehealth is Fueling the Move Towards Microhospitals
Projecting the Supply and Demand for Primary Care Practitioners Through 2020
Are Microhospitals the Answer for Systems Looking for Low-cost Expansions? They Might Be
Microhospitals: Healthcare’s Newest Patient Access Point
Microhospitals Could Prove Financial Boon and Salvation to Healthcare Systems
Microhospitals Provide Health Care Closer to Home
Telemedicine Saves Patients Time and Money, Study Shows
5 Common Questions about Micro-Hospitals, Answered
Survey: More than Half of Patients Prefer Telehealth Visits to In-Person Care
Majority of Parents Plan to Use Telemedicine for Pediatric Care
Microhospitals May Help Deliver Care in Underserved Areas
“Thinking Small” May Be Next Big Innovation in Healthcare Delivery as Microhospitals Spring Up in Metropolitan Areas Across Multiple States










