Feb 16, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Pathology, Management & Operations
Precision medicine programs can benefit from wearable usage data; however, little information has been collected on personalities and behaviors of the device users
Wearables medical devices have the potential to monitor some of the same biomarkers used in medical laboratory tests today. In addition, these mobile technologies can make it possible for clinical laboratories to monitor patients in real time, as well as allow labs to incorporate such into a patient’s historical record of lab test results.
The trend toward personalized medicine (aka, Precision Medicine) is increasing, with many payment programs based on it. Thus, monitoring and correcting activities that cause chronic disease, or work against treatments, is becoming standard procedure for forward-thinking, technically proficient doctors and hospitals. But are patients onboard with all of it?
Activity Trackers for Monitoring Patient Behavior
With the popularity of activity trackers on the rise, researchers are examining their usage patterns to determine how the devices are being utilized, their target market, and ways to encourage sustained use of the gadgets.
A recent article published in Annals of Internal Medicine provided insight regarding who is using this type of wearable device, how activity trackers are being employed, and the length of time consumers will maintain their usage.
The research was spearheaded by Mitesh Patel, MD, Assistant Professor of Medicine and Health Care Management, Perelman School of Medicine and the Wharton School, University of Pennsylvania. He believes this is the largest study of its kind to evaluate the usage of wearable fitness trackers.
“Many people are excited by the potential of using activity trackers to monitor healthy behaviors, but there is very little evidence on who is using them and whether or not use is sustained over time,” Patel stated in a Penn Medicine news release. “We found that, though use grew over time, it really varied depending on individual characteristics like age and income. We also found that once someone started using an activity tracker, sustained use at six months was high at 80%.”

Patel is also Director of the Penn Medicine Nudge Unit, a behavioral design team that is studying the impact that nudges or small interventions may have on healthcare. The team is examining ways in which nudges can influence choices, and also direct medical professionals and patients toward optimal decisions to improve healthcare delivery and results. (Photo copyright: University of Pennsylvania.)
Gaming the Study Improves Usage of Test Devices
To perform the study, 4.4 million members of a national wellness program were invited to take part in data collection. Approximately 55,000 of those individuals actually participated in the study, which involved downloading an app to record pertinent information. Researchers tracked and interpreted the data during a two-year period in 2014 and 2015.
The information analyzed included:
- When participants initially activated their tracker;
- How often the device was utilized;
- The average number of steps taken per day; and,
- Sociodemographic characteristics.
The results of the study were not entirely unexpected, but there were surprises:
- 80% of the people who initially activated the devices were still using them after six months;
- Only 0.2% of the invited individuals used the devices in the first year;
- However, that number increased to 1.2% during the second year.
The usage of wearable activity trackers was nearly double among younger people than it was for older individuals. In addition, people from households with an annual income of less than $50,000 used the gadgets at lower rates than those at higher income levels.
A mere 0.1% of the potential participants were over 65-years old. However, 90% of individuals in this age group were still using the devices six months after initial activation.
The authors of the study stated that adding game elements, such as points, levels, badges and financial incentives may have played a role in the sustained use of the activity trackers.
“Gamification and financial incentives are commonly used within wellness programs, but their impact has not been well studied,” Patel stated in the news release. “Our findings provide initial evidence suggesting that these types of engagement strategies may show promise for keeping sustained use high. However, more studies are needed to determine the best way to combine these types of engagement strategies with activity trackers to improve health outcomes.”
Most Commonly Used Mobile Activity Tracking Devices
There were 60 different types of wearable activity trackers that could be used by participants for the study. Seventy-six percent of those participants elected to use the FitBit activity tracker. This mobile healthcare device is worn on the wrist like a watch. It monitors activity, exercise, food, weight, and sleep to provide consumers with real-time data about their activities.
The data collected by the device is sent automatically and wirelessly to the user’s phone or computer. Individuals then can use the FitBit dashboard to view their progress through online charts and graphs. The dashboard also offers progress notifications to the consumer and gives achievement badges when established goals have been reached.
The second most common activity trackers used were Apple devices, such as Apple Watches, which were chosen by 9% of the participants.
Biometric data on patients’ behavior and activities that is collected and transmitted from mobile devices has swiftly become critical data doctors use in precision medicine diagnoses and treatments. Clinical laboratories will likely be including biomarker data taken by these devices in their testing and procedures in the future. The only question is how quickly the data generated by such devices becomes acceptable to add to a patient’s permanent health record.
—JP Schlingman
Related Information:
New Wellness Study Shows Just How Sticky Wearables Can Be, Even Among Seniors
Penn Study Shows 80% of Activity Tracker Users Stick with the Devices for at Least Six Months
Game Time: To Increase Exercise, Study Shows Gaming Strategies and a Buddy Are Key
When Push Comes to Nudge
Improvements to Fitness Wearables Help Stream Data from Consumers’ Homes to EHRs and Clinical Pathology Laboratories
Apple May Be Developing Mobile Device Technology to Monitor User’s Health and Transmit Data in Real Time
Feb 9, 2018 | Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing
Genalyte’s cloud-based Maverick Detection System could potentially change how and when doctors order blood draws, altering long-standing clinical laboratory workflows
Anatomic pathologists and medical laboratory leaders may be aware of efforts in the in vitro diagnostics (IVD) industry to perform clinical laboratory tests with smaller quantities of blood. The most high-profile company to try and fail is Theranos, which both Dark Daily and its sister print publication The Dark Report reported on as events unfolded.
So far, though, continued efforts to dramatically reduce the amount of blood needed for most typical medical laboratory tests have come up dry. But this has not stopped innovative companies from trying to do so.
One such company is San Diego-based Genalyte. The biomedical diagnostics developer has announced it is readying its new Maverick Detection System (Maverick), which, according to the company’s website, “completes a comprehensive battery of blood tests in the physician’s office with results in 15 minutes.”
According to a news release, “Genalyte is laying the groundwork to move the business of biomedical diagnostics online, with the idea of creating an integrated delivery service for test results that can be generated from a drop of blood.” If successful, Maverick may be poised to disrupt the phlebotomy and clinical laboratory industries in a big way.
Fifteen Minutes from Fingerprick to Clinical Lab Test Results
Maverick, according to its developers, “[will] send digital samples to the cloud for quality review before releasing to the physician and patient. Our central lab handles tests that cannot be completed onsite.
“At the core of our cloud-based, diagnostic laboratory offering is revolutionary technology that uses silicon photonic biosensors to perform multiple tests off a single drop of whole blood in 15 minutes,” notes Genalyte’s website.
In a MedCity News article, Cary Gunn, Genalyte’s founder and CEO, said, “There will always be a need for esoteric testing that needs to be referred to a laboratory. But for the vast majority of routine testing, there’s no reason why that can’t be done in the doctor’s office.”
How Maverick Completes Medical Laboratory Tests in Doctor’s Offices
According to Genalyte’s website, “The Maverick Detection System performs real-time detection of macromolecules in crude samples using biologically functionalized silicon photonic biosensors lithographically printed on disposable silicon chips.”
About the width of a pencil erasure, Maverick biosensor chips “are individually functionalized with unique probe molecules and are individually interrogated, making highly multiplexed analysis possible. As a sample flows over the chip, the probes on the sensors bind with their corresponding ligands. This binding results in a localized change in refractive index on the sensor surface; this change is directly proportional to analyte concentration.”
“The silicon chip itself is watching the chemical reactions take place. Anytime two molecules bind, we can see that happen. So, the technology is capable of almost an infinite number of tests,” Gunn explained in the MedCity News article.
According to the developer, test results are available “in 10-30 minutes depending on the type of assay performed.”

Cary Gunn, PhD, Genalyte’s Founder and Chief Executive Officer, said in a news release that the San Diego-based biomedical diagnostics company wants “to put a rapid and powerful suite of diagnostic tests in every physician’s office.” (Photo copyright: Genalyte.)
Pilot Studies Show Test Feasibility in Doctor’s Offices
The company also announced completion of two pilot studies of the platform’s effectiveness in performing anti-nuclear antibody (ANA) testing. The purpose of study “one” was to “evaluate the feasibility of using this novel instrument to perform ANA 8 tests in the clinic and to compare those results to the same sample tested in Genalyte’s CLIA registered laboratory.” Study “two” focused on “Detection of anti-nuclear antibodies for the diagnosis of connective tissue diseases (CTD).”
The ANA test is often ordered by physicians for diagnosis of CTDs, including:
• Rheumatoid arthritis;
• Systemic lupus erythematosus;
• Raynaud syndrome; and,
• Systemic scleroderma, according to an article in Rheumatoid Arthritis News.
“We are starting with rheumatology, but I call that our entry point,” Gunn told MedCity News. “Our goal is to decentralize the vast majority of diagnostic testing to be near the patient and near the physician.”
The two studies together involved about 750 patients, who were tested by Genaltye’s Maverick system over four months. Results of their blood tests, via fingerprick in the doctor’s office, were compared to traditional medical laboratory procedures and patient diagnoses.
According to the Genalyte video above, “The Maverick Detection System … directly detects the binding of proteins or antibodies to the sensor in real-time and results are analyzed simultaneously with the accompanying Genalyte software. Almost all of the most time consuming and expensive parts of assay development and sample testing are reduced or eliminated.” Click on the image to view the video. (Caption and video copyright: Genalyte.)
According to the news release and the published clinical abstracts, the researchers concluded that:
• Positive and negative results on whole blood tested on the Maverick system highly correlated with serum tested on previously approved devices;
• Multiplex ANA testing on whole blood in physician offices is feasible;
• Venous draw and fingerstick blood samples highly correlated; and
• Maverick has the propensity to improve patients wait times for diagnosis and to enhance their testing convenience.
“There is extremely high correlation for absolute value between venous blood and fingerstick blood, and between positive and negative results seen with whole blood on the Maverick and serum on the FIDIS Connective 10,” noted study “one” researchers.
“I’m impressed,” Patricia Jones, PhD, former President of the American Association for Clinical Chemistry (AACC), told Bloomberg News. “The game-changing part of this would be being able to do testing and potentially make a diagnosis immediately, instead of having to send out lab tests, wait several days, and then call the patient,” she added.
Can One Drop Do It All? Some Researchers Advise Caution
The controversy surrounding point-of-care fingerprick capillary blood draws performed on in-office automated blood analyzers, versus clinical laboratory venous draws performed on high-volume laboratory systems, is not new. Dark Daily has reported on several blood test studies in the past.
One such study involved bioengineers at Rice University. It concluded that fingerpricked capillary blood may not be accurate or reliable enough for clinical decision-making.
Their study acknowledged the value of such capillary blood testing in remote areas. But it also urged caution about use of measurements from a single drop of fingerprick blood.
“Using both a hematology analyzer and POC hemoglobinometer, we found the variability of blood component measures to be greater for successive drops of fingerprick blood than for multiple drops of venous blood,” the researchers wrote in The American Journal of Clinical Pathology (AJCP).
Research will no doubt continue until a viable, accurate, and affordable blood analyzer system that conducts dozens of clinical laboratory tests based on a few drops of blood comes to market. It’s basically inevitable in today’s world where computers can be built from molecules and miniature medical laboratories can be placed in chips, skin patches, and needles.
Pathologists and clinical laboratory leaders would be well advised to monitor the development of these various new diagnostic technologies. For most of the past decade, there has been a steady parade of companies and research teams announcing new discoveries that could revolutionize clinical diagnostics as performed today. However, few disruptive clinical laboratory tests or analyzers based on these technologies have made it into the clinical marketplace.
—Donna Marie Pocius
Related Information:
Can Genalyte Achieve What Theranos Touted?
Genalyte Takes Aim at Lab Testing Giants with Cloud-Based Service
Genalyte Raises $36 Million From Khosla for its One-Drop Blood Test
AACC President Calls Genalyte’s Blood Diagnostic Tests ‘Game-Changing’
Drop-to-Drop Variation in the Cellular Components of Fingerprick Blood: Implications for Point-of-Care Diagnostic Development
Genalyte Diagnostic Tool Shows Potential to Improve Turnaround Time in RA, Other Conditions
Application of a Novel Anti-Nuclear Antibody Multiplex Test Using Finger Stick and Venous Whole Blood in a Rheumatology Clinic—Demonstration of Feasibility
Rice University Researchers Publish Study About Variation in Drop-to-Drop Samples of Capillary Blood Collected by Fingerprick and Used for Clinical Laboratory Testing
After AACC Presentation, Elizabeth Holmes and Theranos Failed to Convince Clinical Laboratory Scientists and the News Media about Quality of Its Technology
Score for Theranos After AACC: Fail
Feb 2, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
Research goal was to isolate circulating tumor cells in venipuncture samples with improved purity compared to standard spiral chips
Many research teams are pursuing the goal of creating assays that detect circulating tumor cells (CTCs) that would allow earlier and more accurate diagnosis of cancer. Now comes news of a unique technology developed at the University of Michigan (U-M) Ann Arbor that showed promised in an early study.
The method of using CTCs to diagnose cancer in patients, while further analyzing specific characteristics of a given cancer case, shows promise as an innovative tool for clinical laboratories and oncologists. However, current approaches face challenges when it comes to proving accuracy and establishing thresholds that might indicate the need for further action.
Researchers at U-M believe they may have solved that problem. They created “Labyrinth,” a “label-free microfluidic device” that condenses 637mm of channels—including 11 loops and 56 corners—onto a 500μm-wide chip that uses inertia and Dean flow to separate white blood cells and CTCs from venipuncture samples at rates as high as 2.5ml per minute. These results improve upon the traditional spiral chip design.
Publishing their findings in Cell Systems, first author of the study Eric Lin, PhD, noted, “With the recent advances in tools for genomic characterization, it is more compelling than ever to look at the tumor heterogeneity to understand tumor progression and resistance to therapies. The Labyrinth device enabled high yields of CTCs without the bias induced by antibody-based selection, allowing the identification of true biological tumor heterogeneity.”
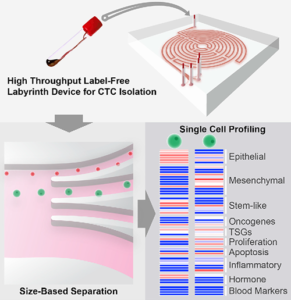
The graphic above, taken from the University of Michigan study, demonstrates the “High-throughput and label-free Labyrinth device that enables single CTC isolation and gene expression characterization.” According to the researchers, “Labyrinth offers a cell-surface marker-independent single-cell isolation platform to study heterogeneous CTC subpopulations.” The U-M study shows promise in creating tools for oncologist and clinical laboratory cancer treatment. (Image copyright: University of Michigan/Cell Systems.)
Challenges in the Isolation of CTCs
The Labyrinth chip is not the first device to assist in isolating CTCs. The U-M study notes that while immune-affinity capture is a validated approach to prognosis, therapeutic monitoring and molecular diagnostics, it does not work with all cancer cases. The researchers also note the method creates challenges in single-cell analysis later.
Existing label-free methods of isolation, such as deterministic lateral displacement, microfluidic flow fractionation, and acoustic-based separation, avoid these concerns but face issues of their own. The researchers noted, “Issues encountered with these approaches include pore clogging, high-pressure drop, pre-fixation to prevent CTC loss, low throughput, and excessive non-specific cell retention.”
The researchers further clarified that a major factor separating the Labyrinth chip from other methods is the ability to identify CTC subpopulations without the need for manual selection based on positive or negative protein expression. Thus, improving the ability to conduct further single-cell analysis from the results. Testing of the Labyrinth chip involved a variety of cancer cell lines, including:
· Human breast (MCF-7);
· Pancreatic (PANC-1);
· Prostate (PC-3); and,
· Lung (H1650).
And while standard spiral chips are already a common method for conducting size-based sorting, the purity of results is less than ideal with thousands of other cells remaining in the sample.
The researchers reported that the Labyrinth chip recovered 91.5% (plus or minus 0.9%) of cancer cells and removed 91.4% (plus or minus 3.3%) of white blood cells in a spiked buffer test.
“Bigger cells, like most cancer cells, focus pretty fast due to the curvature. But the smaller the cell is, the longer it takes to get focused,” Sunitha Nagrath, PhD, Associate Professor of Chemical Engineering and a lead developer of the Labyrinth chip, stated in a U-M news release. “The corners produce a mixing action that makes the smaller white blood cells come close to the equilibrium position much faster.”
Labyrinth also supports a series configuration of multiple chips. While testing two chips in series, researchers noted “a two-log improvement in tumor cell enrichment over the single Labyrinth.” They claim this is a higher purity than other label-free methods they studied, while adding only five minutes to processing times.

Sunitha Nagrath, PhD (above), is an Associate Professor of Chemical Engineering at the University of Michigan, and one of the lead developers of the Labyrinth chip. “You cannot put a box around these cells,” she noted in the U-M news release. “The markers for them are so complex, there is no one marker we could target for all these stages.” (Photo copyright: University of Michigan.)
Current Testing Using the Labyrinth Chip
The chip is already in use in a clinical trial for an aggressive form of breast cancer by Max Wicha, MD, Madeline and Sidney Forbes Professor of Oncology, Founding Director Emeritus, University of Michigan Comprehensive Cancer Center, and co-author of the Cell Systems study, who lead the study along with Nagrath.
The trial involves the attempted activation of adult system cells by blocking the signaling molecule interleukin-6. Wicha suspects the molecule enables cancer stem cells as well. “We think that this may be a way to monitor patients in clinical trials,” he said in the U-M news release. “Rather than just counting the cells, by capturing them, we can perform molecular analysis [to] know what we can target with treatments.”
The news release further highlights how this chip is specifically suited to such a task. As cancer stem cells transition from stem-like cells to more ordinary cell types, their gene expression shifts as well. This creates an issue when using conventional cell targeting. Nagrath notes this concern, stating, “The markers for [cancer stem cells] are so complex, there is no one marker we could target for all these stages.”
The Labyrinth chip shows potential for overcoming one of the biggest hurdles to leveraging CTCs to diagnose cancers and develop personalized therapies. Currently, the chip can output to Fluidigm, DEPArray by Silicon Biosystems, and RainDance Technologies’ RainDrop Digital PCR System.
The U-M researchers hope that future research will yield additional applications and compatible systems to further improve the ability for medical laboratories to use CTCs in the early detection and monitoring of cancer cases.
—Jon Stone
Related Information:
‘Labyrinth’ Chip Could Help Monitor Aggressive Cancer Stem Cells
High-throughput Microfluidic Labyrinth for the Label-free Isolation of Circulating Tumor Cells
Novel Labyrinth Chip Monitors Cancer Stem Cells in Clinical Trial
‘Labyrinth’ Device Sorts Cancer Cells from Healthy Blood
This Awesome Blood Labyrinth Is the Newest Method for Catching Cancer Cells
Labyrinth Chip Has the Potential to Lead to Customized Cancer Treatments
Jan 31, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing
Researchers are finding multiple approaches to metabolomic research and development involving disparate technology platforms and instrumentation
Human metabolome has been discovered to be a wealth of medical laboratory biomarkers for diagnosis, therapy, and patient monitoring. Because it can provide a dynamic phenotype of the human body, there are many potential clinical laboratory applications that could arise from metabolomics, the study of metabolites.
Researchers are discovering numerous ways the expanding field of metabolomics could transform the future of healthcare. However, to fully exploit the potential of human metabolome, developers must choose from various approaches to research.
“The metabolites we’re dealing with have vast differences in chemical properties, which means you need multi-platform approaches and various types of instrumentation,” James MacRae, PhD, Head of Metabolomics at the Francis Crick Institute in London, told Technology Networks. “We can either use an untargeted approach—trying to measure as much as possible, generating a metabolic profile—or else a more targeted approach where we are focusing on specific metabolites or pathways,” he added.
A multi-platform approach means different diagnostic technologies required to assess an individual’s various metabolomes, which, potentially, could result in multi-biomarker assays for medical laboratories.
Measuring All Metabolites in a Cell or Bio System
Metabolomics is the study of small molecules located within cells, biofluids, tissues, and organisms. These molecules are known as metabolites, and their functions within a biological system are cumulatively known as the metabolome.
Metabolomics, the study of metabolome, can render a real-time representation of the complete physiology of an organism by examining differences between biological samples based on their metabolite characteristics.
“Metabolomics is the attempt to measure all of the metabolites in a cell or bio system,” explained MacRae in the Technology Networks article. “You have tens of thousands of genes, of which tens of thousands will be expressed—and you also have the proteins expressed from them, which will then also be modified in different ways. And all of these things impact on a relatively small number of metabolites—in the thousands rather than the tens of thousands. Because of that, it’s a very sensitive output for the health or physiology of your sample.
“With that in mind, metabolomics has great potential for application in most, if not all, diseases—from diabetes, heart disease, cancer, HIV, autoimmune disease, parasitology, and host-pathogen interactions,” he added.

The graphic above is taken from a study published in the Journal of the American College of Cardiology (JACC). It notes, “State-of-the-art metabolomic technologies give us the ability to measure thousands of metabolites in biological fluids or biopsies, providing us with a metabolic fingerprint of individual patients. These metabolic profiles may serve as diagnostic and/or prognostic tools that have the potential to significantly alter the management of [chronic disease].” (Image and caption copyright:Journal of the American College of Cardiology.)
• Genomics: the study of DNA and genetic information within a cell;
• Proteomics: the large-scale study of proteins; and,
• Transcriptomics: the study of RNA and differences in mRNA expressions.
Researchers caution that metabolomics should be used in conjunction with other methods to analyze data for the most accurate results.
“Taking everything together—metabolic profiling, targeted assays, label incorporation and computational models, and also trying to associate all of this with proteomics and
genomics and transcriptomic data—that’s really what encapsulates both the power and also the challenges of metabolomics,” MacRae explained.
Metabolome in Precision Medicine
Metabolomics may also have the ability to help researchers and physicians fine-tune therapies to meet the specific needs of individual patients.
“We know we’re all very different and we don’t respond to drugs in the same way, so we could potentially use metabolomics to help select the best treatment for each individual,” Warwick Dunn, PhD, Senior Lecturer in Metabolomics at the University of Birmingham, Director of Mass Spectrometry, Phenome Center Birmingham, and, Co-Director, Birmingham Metabolomics Training Center, UK, told Technology Networks.
“Our genome is generally static and says what might happen in the future. And the metabolome at the other end is the opposite—very dynamic, saying what just happened or could be about the happen,” Dunn explained. “So, we could apply it to identify prognostic biomarkers, for example, to predict if someone is at greater risk of developing diabetes five to ten years from now. And if you know that, you can change their lifestyle or environment to try and prevent it.”
Metabolomics continues to tap the many diagnostic possibilities posed by the human metabolome. And, the resulting human biomarkers derived from the research could result in a rich new vein of medical laboratory assays.
—JP Schlingman
Related Information:
Metabolomics and Health: On the Cusp of a Revolution
‘Metabolomics’ Distinguishes Pancreatic Cancer from Pancreatitis
Using Metabolomics to Prevent Colon Cancer
Applications of Metabolomics
The Emerging Role of Metabolomics in the Diagnosis and Prognosis of Cardiovascular Disease
Metabolomics Takes Another Step Forward as Methodology for Clinical Laboratory Testing with Development of an Assay for the Diagnosis of Concussion
Jan 26, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Sales and Marketing, Laboratory Testing, Management & Operations
Lack of Medicare or third-party payer coverage for most genetic screening tests in healthy adults is not discouraging development of new gene testing products
With the global anatomic pathology genetic testing market poised to reach $9.8 billion by 2025, clinical laboratories continue to develop new genetic screening tests (rather than diagnostic tests) intended to help physicians identify patients who carry inherited genetic mutations that could put them or their future children at higher risk for chronic disease, such as cancer.
This is a bit of a gamble since (with some exceptions) Medicare and many health insurers typically will not pay for predictive and presymptomatic genetic tests and services used to detect an undiagnosed disease or disease predisposition.
Nevertheless, Inkwood Research of Gurugram, India, predicts in its “Global Genetic Testing Market Forecast 2017-2024” report that aging populations throughout the world will be the driving force producing “enormous opportunities for the global genetic testing market.” The research firm anticipates this will result in a 9.93% increase in annual sales revenue during each of the next seven years.
Screening versus Diagnostic Testing Gains Popularity Among Patients, Physicians
Genetic diagnostic testing promises to accelerate the growth of precision medicine by guiding the diagnosis and treatment of cancer and other chronic diseases. However, genetic tests that “screen” healthy patients for predispositions to certain diseases also are gaining traction in the marketplace.
The US Food and Drug Administration (FDA) gave direct-to-consumer genetic screening testing a boost in April 2017 when it allowed marketing of 23andMe Personal Genome Service Genetic Health Risk tests for 10 inherited diseases or conditions, including:
· Parkinson’s Disease;
· Late-onset Alzheimer’s Disease;
· Celiac Disease; and
· other conditions.
“Consumers can now have direct access to certain genetic risk information,” Jeffrey Shuren, MD, Director of the FDA’s Center for Devices and Radiological Health, said in a press release. “But it is important that people understand that genetic risk is just one piece of the bigger puzzle, it does not mean they will or won’t ultimately develop a disease.”
Robert Green, MD, MPH, a Professor of Medicine at Harvard Medical School, told NPR that consumers should have access to genetic information. However, they also need to understand its limitations.
“Some people really want this [genetic] information on their own, and others want it through their physician,” Green said. “Both those channels are legitimate. People should just be aware that this information is complicated.”
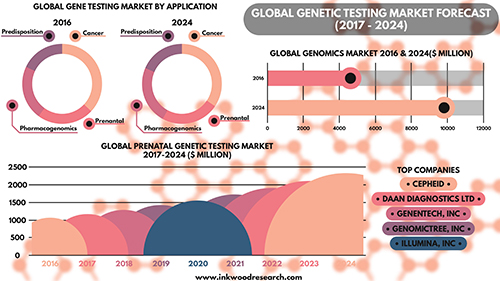
According to the Inkwood Research report, “The global genetic testing market is anticipated to grow from $4,614 million in 2016 to $9,806 million by 2025, at a CAGR [Compound Annual Growth Rate] of 9.93% between 2017 and 2025. The important driver increasing growth in the global genetic testing market is an aging population on the rise. The rising geriatric population is driving the global genetic testing market to a significant level.” (Caption and graphic copyright: Inkwood Research.)
· Cystic Fibrosis;
· Sickle Cell Disease; and
· Spinal Muscular Atrophy.
The genetic screening panel tests for the 22 heritable diseases cited by the American College of Obstetricians and Gynecologists (ACOG) in a Committee Opinion on genetic carrier screenings published by the ACOG in March 2017.
“The United States is truly a melting pot, and it no longer makes sense for physicians to assume genetic screening is appropriate for an individual based on presumed race or ethnicity,” Felicitas Lacbawan, MD, Executive Medical Director, Advanced Diagnostics, Quest Diagnostics, stated in a press release. “QHerit is designed for any woman and her partner, not just those in a specific, so-called high-risk ethnic or racial group.”
Genetic Screening in Primary Care Helps Assess Risk for Chronic Disease
Genetic diagnostic test developer Invitae (NYSE:NVTA) also points to growing evidence of the genetic screening test’s value to healthy individuals. In September 2017, Invitae presented initial findings at the National Society of Genetic Counselors 36th Annual Conference. The study showed a retrospective analysis of 120 patients tested with a proactive genetic screening panel for healthy adults had revealed medically significant findings for nearly one in five patients.
“Interest among otherwise healthy adults in using genetic information to understand their risk of disease conditions continues to grow each year, ” Robert Nussbaum, MD, Chief Medical Officer of Invitae, said in a press release. “These and other data show that interest is well-placed, with a substantial group of patients showing genetic variants associated with elevated risk of diseases like cancer where monitoring and early intervention can be helpful. Use of genetic screening in the primary care setting can assess risk to help shape individual screening plans. We are continually adding tools and resources that help reduce barriers to the widespread use of genetic information in mainstream medical practice.”
Routine Genetic Screening Could Become Norm, CDC Says
The Centers for Disease Control and Prevention (CDC) notes that newborn screening is “currently the largest public health genetics program in the world,” with more than four million babies screened at birth each year for 30 or more genetic conditions. In the CDC’s “Genomics and Health Impact Blog,” the agency continues to maintain a “cautionary attitude about personal genomic tests” beyond the newborn period, directing those considering direct-to-consumer laboratory testing, such as 23andMe and MyMedLab, to “think before you spit.”
Nonetheless, the CDC acknowledges routine genetic screening of healthy people could become the norm. However, others advise caution.
“To be sure, while the use of genome sequencing is promising in certain clinical scenarios, such as rare diseases and cancer, we do not think that whole genome sequencing in the general population is appropriate at this time,” Muin J. Khoury, PhD, MD, Director, Office of Public Health Genomics, CDC, wrote in a January 30, 2017, blog post. “We would not recommend its use outside research studies … But it is also becoming clearer that as science progresses, we are discovering more opportunities for using genetic screening of healthy individuals for preventing common diseases across the lifespan, outside of the newborn screening context.”
The impact on clinical laboratories and anatomic pathology groups should genetic screening become normalized should be clear: Labs will be tasked with performing these tests, and pathologists will be needed to interpret them and educate both physicians and patients on the findings.
Before that, however, genetic screening tests will need to be fully supported by government, and insurers, including Medicare, will have to agree to pay for them.
—Andrea Downing Peck
Related Information:
Global Genetic Testing Market Forecast 2017-2024
Carrier Screening for Genetic Conditions
Quest Diagnostics Launches QHerit, a Pan-Ethnic Genetic Screening Panel Aligned with New Medical Guidelines
Invitae Expands Test Menu for Proactive Genetic Testing in Healthy Adults
Invitae Highlighting New Research, Expanded Suite of Services at National Society of Genetic Counselors (NSGC) 36th Annual Conference
Consumer Genetic Testing: Think Before You Spit, 2017 Edition
Genetic Screening of Healthy Populations to Save Lives and Prevent Disease
FDA Allows Marketing of First Direct-to-Consumer Test that Provide Genetic Risk Information for Certain Conditions
FDA Approves Marketing of Consumer Genetic Tests for Some Conditions
Jan 19, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
This may especially benefit cancer research and treatment thanks to MALDI’s ability to provide pathologists with a view of the whole-tissue micro-environment
Though it may be years before Matrix-Assisted Laser Desorption Ionization (MALDI) mass spectrometry finds use in clinical applications, recent developments show medical laboratories and anatomic pathologists how one type of technology is being rapidly adapted for use in diagnosing cancers.
Richard Drake, PhD, Director of the Medical University of South Carolina (MUSC) Proteomics Center, notes the importance of MALDI to cancer research. “In the clinic, there has to be something that will facilitate looking at all this data—tools that will let the pathologists look at it as well as the mass spec person,” Drake told GenomeWeb.
“It has been known for decades that glycosylation changes on the cell surface promotes cancer progression and the way the immune system sees a tumor or doesn’t see a tumor,” he explained. “That’s the advantage of MALDI imaging. You’re looking at the whole tissue micro-environment, and particularly for cancer it turns out to be important.”
Imaging Mass Spectrometry Applications for Anatomic Pathology
MALDI uses mass spectrometry imaging technology to enable high-molecular identification and an overall view of tissue. It differs from liquid chromatography-mass spectrometry (LC-MS), which is a chemical analysis technique.
An article by News-Medical describes in detail how MALDI technology works:
“MALDI imaging works through the utilization of a matrix, an acidic aromatic molecule that absorbs energy of the same wavelength produced by the irradiating laser. The matrix transfers the substance being examined to the gas state, thereby producing ionization in a three-step process:
1. “Thin sample sections on a metal slide are first covered with the matrix and the procedure for extracting molecules of interest from the tissue into the matrix begins. The matrix can be applied both manually and automatically.
2. “The laser irradiates the sample only in the matrix layer, meaning the underlying tissue remains intact.
3. “The released molecules are transferred to the gas state as the matrix absorbs the laser energy. Ions are formed due to the addition or removal of protons when in the gas state.
“The irons are required for further analysis via the mass spectrometer. The metal slide is placed into a MALDI mass spectrometer where the spatial distribution of the biological molecules is mapped. Within the mass spectrometer, the tissue specimen is raster scanned forming a mass spectrum for each spot measured. Image processing software is then required to import the data from the mass spectrometer to allow visualization of the image produced.”

The above schematic illustrates “the identification of bacteria and yeast by MALDI-TOF MS using the intact-cell method. Bacterial or fungal growth is isolated from plated culture media (or can be concentrated from broth culture by centrifugation in specific cases) and applied directly onto the MALDI test plate. Samples are then overlaid with matrix and dried. The plate is subsequently loaded into the MALDI-TOF MS instrument and analyzed by software associated with the respective system, allowing rapid identification of the organism.” (Caption and image copyright: Clinical Microbiology Reviews/American Society for Microbiology.)
MALDI in Clinical Laboratories
MALDI experts at MUSC worked with researchers at Bruker Corporation, a developer of scientific instruments and analytical diagnostic solutions for cell biology, preclinical imaging, clinical phenomics and proteomics research, clinical microbiology, and for molecular pathology research. Bruker is reportedly working with labs in Europe on MALDI-based assays for clinical use.
Developing MALDI applications for use in clinical laboratories and anatomic pathology groups could result in major improvements. Imaging mass spectrometry could:
- make more molecular information available;
- reduce pathology’s subjectivity and intra-observer nature;
- enable more accuracy and ability to duplicate current pathology assays; and,
- pave the way for new assays to be made.
“MALDI-IMS [imaging mass spectrometry] identifies the distributions of proteins, peptides, small molecules, lipids, and drugs and their metabolites in tissues, with high spatial resolution. This unique capacity to directly analyze tissue samples without the need for lengthy sample preparation reduces technical variability and renders MALDI-IMS ideal for the identification of potential diagnostic and prognostic biomarkers and disease gradation,” noted authors of a MALDI study published in the July 2017 edition of Biochimica et Biophysica Acta Proteins and Proteomics.
“You can take a slide of tissue and essentially do metabolomics on it so that you can look at the intricate nature of what metabolism is happening within a tissue,” James MacRae, PhD, Head of Metabolomics at the Francis Crick Institute in London, told Technology Networks, which described development of new mass spectrometry imaging technologies as “potentially game-changing.”
Mass Spectrometry in Clinical Laboratories
This is just the latest in a string of scientific developments involving mass spectrometry over the past decade that are potential boons to clinical laboratories. In “Is Mass Spectrometry Ready to Challenge ELISA for Medical Laboratory Testing Applications?” Dark Daily reported on the development of a new technique from the Department of Energy’s Pacific Northwest National Laboratory that uses mass spectrometry to identify protein biomarkers associated with cancer and other diseases. Researchers dubbed the technique PRISM, which stands for Proteomics Research Information System and Management.
And in “Swiss Researchers Use New Mass Spectrometry Technique to Obtain Protein Data, Create Strategy That Could Lead to Clinical Laboratory Advances in Personalized Medicine,” Dark Daily reported on researchers at the Swiss Federal Institute of Technology in Lausanne and ETH Zurich who developed a new way to use mass spectrometry to explain why patients respond differently to specific therapies. The method potentially could become a useful tool for clinical laboratories that want to support the practice of precision medicine.
As mass spectrometry’s role in clinical laboratories continues to expand, MALDI technology development and research could eventually lead to tools and applications that enhance how anatomic pathologist view tissue specimens in the medical laboratory. Though the research is ongoing, the technology seems particularly suited to cancer research and treatment.
—Donna Marie Pocius
Related Information:
Technical Advances Position MALDI Imaging as Plausible Tool for Clinical Pathology
Bruker Introduces Novel Mass Spectrometry Solutions for MALDI Imaging, Metabolomics, Proteoform Profiling, and Toxicology at ASMS 2017
The Proteomics of Prostate Cancer Exosomes
MALDI Imaging
Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry: A Fundamental Shift in the Routine Practice of Clinical Microbiology
Metabolomics and Health – On the Cusp of a Revolution
Is Mass Spectrometry Ready to Challenge ELISA for Medical Laboratory Testing Applications?
Swiss Researchers Use New Mass Spectrometry Technique to Obtain Protein Data, Create Strategy That Could Lead to Clinical Laboratory Advances in Personalized Medicine
Precision Medicine Summit Feb. 21, 2018











