Jan 17, 2018 | Coding, Billing, and Collections, Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Pathology, Laboratory Testing, Management & Operations
Moving to market are the newest generation of LIMS products designed to serve clinical laboratories while supporting quality reporting initiatives and new sources of revenue
It was Bob Dylan who made a big hit out of the song, “The Times, They Are A-Changin’.” The same could be said for the next generation of software products designed for use by medical laboratories.
To be fully successful, these next-generation laboratory information management systems (LIMS) must be radically different than the generations that came before. For example, medical laboratories are frustrated with the many limitations of older LIS products that still incorporate software technologies that date back to the 1980s and 1990s, such as MUMPS, which stands for Massachusetts General Hospital Utility Multi-Programming System.
But the newest LIMS products must do more than simply incorporate the latest technologies in software and cloud-based services. They must support all the ways that clinical laboratories and anatomic pathology groups generate increased revenue. More specifically, all medical laboratories will be generating vast quantities of molecular and genetic data. Therefore, an effective LIMS must be capable of capturing that data while also enabling the lab to perform certain healthcare big data analyses in support of the referring physicians and parent hospitals.
There also will be the need for medical laboratories to use their LIMS capabilities to support the data reporting requirements of Medicare and private health insurers. Payers increasingly want providers to report their quality monitoring, patient outcomes, and certain cost-of-care parameters. All these are functions that older LIS (laboratory information systems) products were not developed to provide.
Anatomic pathology group stakeholders and clinical laboratory managers understand the vital importance of their LIMS. Laboratory and healthcare workflows depend on the system’s:
- efficiency;
- scalability that supports the growth of the lab and medical practice; and,
- flexibility to interface with modern, point-of-care telehealth technologies in ways that enable labs and practices to engage in today’s precision medicine healthcare initiatives.
The more immediate need is for a LIMS to be capable of supporting Medicare’s Quality Payment Programs (QPPs), primarily the MACRA Merit-based Incentive Payments System (MIPS). Most physicians, including pathologists, will participate in MIPS. The first Medicare incentives or penalties will be paid next year, based on 2018 metrics and performance.
Given all these changing demands of advanced software technologies and the need for medical laboratories to participate in various value-based revenue programs, how might a LIMS empower labs to ensure success and increased revenue?
Quality Payment Programs and Merit-based Incentives
As part of the shift toward value-based care, the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) works to drive down costs and increase quality within both care and laboratory environments. MACRA establishes a data-driven payment system to reimburse service providers based on the outcome of services and care episodes, instead of the volume of services delivered or billed.
Combined with reduced payments, MACRA’s incentives and penalties, and Medicare’s QPP/MIPS payment programs, pressure has been increased on healthcare providers and medical laboratories alike. Thus, technology that gives labs a competitive edge is essential for thriving in an ever-evolving and increasingly competitive marketplace.
Meeting MACRA Goals with a Laboratory Information Management System
While electronic health record (EHR) systems have helped to consolidate patient protected health information (PHI), they do little to address the real-time creation of laboratory data and the accessibility of the massive volume of lab-related data stored in the average patient’s medical files.
A LIMS, however, helps to consolidate all this data in an easily accessible and powerful system. Some LIMS even combine with telehealth technologies to make data actionable and available at the point-of-care.
In this type of LIMS, laboratories, physicians, and other care providers all access the same dataset to ensure information is relayed quickly and efficiently. Interaction takes place using cloud-based interfaces, such as mobile apps or web portals. This ensures access to patient data and laboratory test results in a variety of locations without dependence on proprietary communications systems or hardware.
From bustling ERs and surgical wards to phlebotomists visiting long-term care facilities and mobile clinics, collecting and retrieving data becomes streamlined and accessible virtually anywhere.
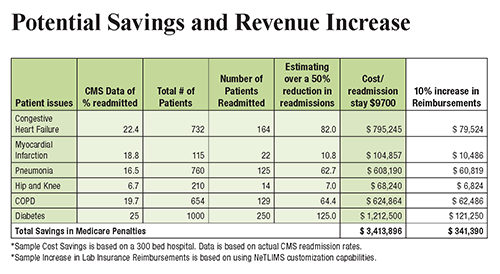
The chart above illustrates how a LIMS offers increased potential to automate processes and scale operations while keeping physicians, patients, and other critical parties up to date. This increase in efficiency and access to data empowers providers to reach improved patient outcomes and reduce hospital readmission rates, increasing revenue for both clinicians and clinical laboratories. (Graphic copyright: NetLIMS.)
When implemented properly, a LIMS also helps laboratories and healthcare facilities meet the terms of MIPS. This reduces Medicare penalties and ensures payment adjustments, which improve revenue streams even further.
Understanding LIMS and Cloud-Based Lab Systems
To help outline and explain the benefits of a LIMS for laboratories and healthcare facilities, The Dark Report, in conjunction with NetLIMS, a global provider of laboratory information management systems to hundreds of hospitals and commercial laboratories worldwide, has produced a free white paper titled, “The Path to More Revenue: Cloud-Based LIMS, Mobile Apps, and Point-of-Care Telehealth.”
- This white paper addresses critical concerns, including:
- Overviews of new technologies;
- The impact of value-based programs on the lab market;
- The importance of MACRA and MIPS adherence;
- How technology, such as a LIMS, can help labs achieve improved efficiency; and,
- Tips on choosing a LIMS vendor to maximize ROI.

To download your free copy of the whitepaper click on this link: Or, copy this URL into your browser: https://www.darkdaily.com/whitepaper/the-path-to-more-revenue-cloud-based-lims-mobile-apps-and-point-of-care-telehealth .
Thanks to advances in LIMS design and development, remote patient digital therapeutics, and cloud-based technology, healthcare providers now have unprecedented opportunities to better manage the health of patients with chronic conditions. In addition, it can help you achieve better efficiency, economics, and compliance with MACRA.
This free white paper is your first step toward significantly reducing hospital readmission rates, bridging the gap between labs, physicians, and other healthcare providers they serve, and positively affecting patient outcomes, improving quality measures, and maximizing reimbursements for all services you provide.
—Jon Stone
Related Information:
The Path to More Revenue: Cloud-Based LIMS, Mobile Apps, and Point-of-Care Telehealth
How Close Is the End of Private Practice Pathology as We’ve Known It?
Attention Anatomic Pathologists: Do You Know Medicare Is Prepared to Change How You Are Paid, Beginning on January 1, 2017?
Innovator Hospitals Bring ICUs into the Info Age, Using New Design Approaches that involve Medical Laboratory Tests
Jan 15, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
Lab-on-skin is the latest concept to join the lab-on-a-chip, lab-in-a-needle, and lab-on-paper field, as researchers continue to seek ways to miniaturize medical laboratory tests
Move over, lab-on-a-chip and lab-on-paper. There’s a new diagnostic technology in research labs that is gaining credibility. It is called lab-on-skin technology and some scientists are quite excited about how it might be used for a variety of clinical purposes.
A recent story published in ACS Nano titled, “Lab-on-Skin: A Review of Flexible and Stretchable Electronics for Wearable Health Monitoring,” reviews the latest advancements in lab-on-skin technology. It provides an overview of different research initiatives incorporating lab-on-skin technologies.
From telehealth to precision medicine to point-of-care mobile devices, anatomic pathologist and clinical laboratories are about to be challenged with new diagnostic technologies. These technologies are intended to streamline the workflow between physicians and medical laboratories while improving access to patient data and medical laboratory test results.
Of all the mobile devices designed to support medical care, no technology may have more potential to change the pathology profession than nanotechnology-based diagnostic devices. Whether lab-on-a-chip, lab-in-a-needle, or lab-on-paper, these miniature laboratories are so small dozens can be carried in a pocket.
Most importantly, for certain diagnostic tests, some of these devices being developed hope to deliver full-size-lab quality results accurately and inexpensively, even in rural regions and areas with little or no resources, such as electricity or water. (See Dark Daily, “Lab-on-a-Chip Diagnostics: When Will Clinical Laboratories See the Revolution?” September 9, 2016.)
Now, researchers have demonstrated that even biomarkers within human skin can be tested by medical wearable devices. “Lab-on-skin” has entered the pathology vernacular.
Lab-on-Skin Constantly Measures Physiological Data
According to ACS Nano, lab-on-skin devices are small electronic patches worn directly on the skin that noninvasively measure a variety of physiological data. These flexible gadgets can interpret information including:
- body temperature;
- blood oxygenation;
- hydration;
- blood pressure;
- glucose;
- potassium;
- sodium; and,
- lactate and pH levels in individuals.
The devices may also be used for wound care, prosthetics and rehabilitation, as well as for optogenetics and human-machine interfaces (HMI).
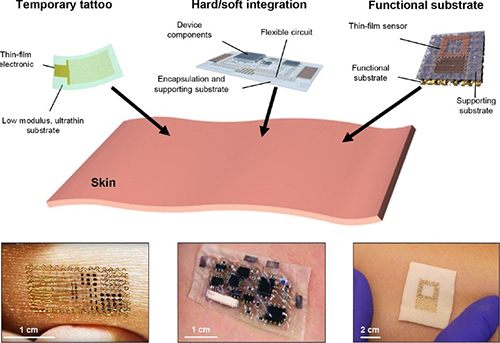
The image above from the ACS Nano article demonstrates various lab-on-skin devices, including: an NFC tattoo with a bare die chip mounted on an acrylic adhesive film; a soft radio sensor with commercial chips encapsulated in a fluid/ecoflex package; and, a sweat sensor on silicone foam. Each of these devices could be capable of delivering actionable diagnostic data to anatomic pathologists and clinical laboratories. (Image copyright: ACS Nano.)
Lab-on-skin technology can be utilized to read electrophysiological signals typically measured by electrodes placed on various parts of the body, such as:
The direct connection between the patches and the skin allows for continuous and precise data collection without the threat of drying out that comes with traditional electrodes.
Nanotechnology Driving Clinical Laboratory Diagnostic Applications
Because it is the largest organ in the body, skin provides a perfect pathway to convey biological information originating from various parts of the body, such as inner organs, muscles, blood vessels, and the dermis and epidermis.
The ACS Nano article discusses advancements in the designs and materials used for lab-on-skin patches. In addition to the term “lab-on-skin,” these devices may also be referred to as electronic skin, epidermal electronics, and electronic tattoos. They have untapped potential in a variety of clinical applications, including:
For example, researchers at the University of Illinois at Urbana-Champaign have created an epidermal nanotechnology device that utilizes sensors and wireless interfaces to measure ultraviolet (UV) exposure, a risk factor for skin cancers.
“Our goal with this research is to establish a set of foundational materials and device designs for systems that can improve health outcomes by providing information on UV exposure,” John A. Rogers, PhD, and Professor of Materials Science and Engineering and Professor of Chemistry told Nanowerk Spotlight.
Nanotechnology employs extremely small particles performed at the nanoscale (about 1 to 100 nanometers). This field is emerging as a vital element behind cutting-edge innovations in medicine and healthcare.
“We developed new chemistries that yield color changes that quantitatively relate to total exposure dose, separately in both the UV-A and UV-B regions of the solar spectrum,” explained Rogers. “Our formulations have the additional advantage that they provide soft, low modulus mechanics to enhance comfort and biocompatibility with the skin surface.”
Mini-Laboratory Devices Could Push Pathology Data to Clinical Laboratories
The combination of using lab-on-skin devices with nanotechnology can provide researchers and medical professionals a multifunctional and valuable tool for health monitoring and the diagnosis of diseases. However, more research and clinical studies are needed to establish the validity of using lab-on-skin devices in healthcare applications.
Nevertheless, clinical laboratories and pathology groups will be handling more data in the future, generated by these miniature laboratory devices. Their usefulness, especially in challenging healthcare environments, is only beginning to be fully discovered.
—JP Schlingman
Related Information:
A Lab-on-Skin: A Review of Flexible and Stretchable Electronics for Wearable Health Monitoring (downloadable PDF)
Lab-on-Skin: A Review of Flexible and Stretchable Electronics for Wearable Health Monitoring (original ACS Nano article)
Lab-on-Skin: Nanotechnology Electronics for Wearable Health Monitoring
Stick-on Epidermal Electronics Tattoo to Measure UV Exposure
Nanotechnology in Healthcare (Part 1: Fitness Monitoring, Diagnostics and Prevention)
Nanotechnology in Healthcare (Part 2: Nanomedicine Therapy)
Breathable, Wearable Electronics on Skin for Long-term Health Monitoring
Nano-chip Promises to Heal Organs at a Touch
IBM and Mount Sinai Researchers Develop Innovative Medical Lab-on-a-Chip Solution
Lab-on-a-Chip Diagnostics: When Will Clinical Laboratories See the Revolution?
Researchers at University of Rhode Island Unveil Lab-on-Paper Test Capable of Multireagent Diagnostics: Could Enable ‘Diagnostics Without the Lab’ Say Developers
Sleek ‘Lab in a Needle’ Is an All-in-One Device That Detects Liver Toxicity in Minutes during a Study, Showing Potential to Supplant Some Medical Laboratory Tests
Jan 10, 2018 | Compliance, Legal, and Malpractice, Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory News, Laboratory Pathology, Laboratory Testing
CMS sends letter to Orig3n notifying the genetic test company that it may not have the required certifications to market its genetic tests
Orig3n’s recent ill-fated “DNA Day” promotion to offer free genetic tests during an NFL football game this past fall pushed Orig3n into the media spotlight. The Massachusetts-based biotech company—which sells 18 different DNA tests on its website—suspended the promotion due to questions from the Centers for Medicare and Medicaid Services (CMS) and the Maryland Department of Health (MDH) regarding the legality of the testing under the Clinical Laboratory Improvement Amendments of 1988 (CLIA).
Since then, however, new details from BuzzFeed and GenomeWeb indicate that Orig3n may not have the required certifications to market their genetic tests after all. On October 30, 2017, CMS served Orig3n with an out-of-compliance notice. According to BuzzFeed, the letter came from Karen Dyer, MT (ASCP) DLM, Director, Division of Laboratory Services and the CLIA program at CMS.
In a letter to Kate Blanchard, Chief Operating Officer at Orig3n, Dyer wrote, “To apply for CLIA certification, Orig3n must contact both the Massachusetts and California state agencies immediately for guidance. Orig3n’s various tests analyze 18 genes related to health, from ‘muscle power’ to ‘sugar sensitivity’ to ‘age-related metabolism’. It offers genetic testing that provides information for the assessment of health.” The letter gave Orig3n a November 13 deadline to update CMS on issues regarding their CLIA certification.
Robin Smith, CEO, Orig3n, told GenomeWeb the notice “was the first time that any clear guidance was given regarding specific genes and requirements for CLIA/non-CLIA.” He also noted efforts Orig3n undertook over the prior year to fully certify their laboratory.

The test shown above is one of 18 genetic tests Orig3n offers direct to consumers. According to Vice, Orig3n claims their tests do not require FDA-approval “because the tests are not diagnostic [and] they don’t require it.” The Baltimore Sun reported that “Orig3n is confident it can receive the proper approvals and plans to have a fan giveaway later this season at one of our games.” (Photo copyright: Orig3n.)
Fortunately for Orig3n, meeting compliance and obtaining certification for their existing lab is no longer a requirement to resolve the issue. In a November press release, Orig3n announced the purchase of Interleukin Genetics. Orig3n plans to absorb Interleukin’s existing assets, including a CLIA-certified genetics laboratory in Waltham, Mass., capable of analyzing more than one million samples annually.
“Once we met with Interleukin Genetics, we saw a natural alignment between the two organizations regarding our shared commitment to a future of personalized health,” Smith noted. “With our trajectory of accelerated growth, we couldn’t imagine a better fit for acquisition. We are very pleased to be welcoming Interleukin Genetics to Orig3n.”
GenomeWeb asked Blanchard how the acquisition would impact Orig3n’s commercialization of the 18 tests in question by CMS, now that Orig3n owns a CLIA-certified lab, and through it, meets the requirements of CMS’ out-of-compliance notice. Blanchard declined to comment.
New Concerns Surrounding Interleukin Assets
Yet, in solving one set of problems, some experts believe Orig3n might have inherited a new set. In July 2016, GenomeWeb reported that Interleukin Genetics would be laying off 63% of its staff. Unable to secure a clinical services agreement, the company could not extend debt payment deferrals with its senior lenders. At the time of writing, debts totaled $5.6 million.
Further complicating matters, a 2015 peer-reviewed analysis published in the Journal of the American Dental Association (JADA) questioned the clinical validity of an inflammation management program called “Ilustra” that Interleukin claimed, “identifies individuals with an increased risk for severe and progressive periodontitis, due to a life-long genetic predisposition to over-produce Interleukin-1 (IL-1), a key mediator of inflammation.”
Another GenomeWeb article reported on the turbulent road the Ilustra program followed until Orig3n eventually pulled it from the market. GenomeWeb noted critics’ concerns about the marketing of precision medicine, genetic testing, and regulatory issues facing medical laboratories as these technologies mature.
Clinical Laboratories Continue to Field Concerns Over DTC Testing
“This [genetic] test would have been laughed out of the room if it had been presented to oncologists, or to professionals in medical genetics,” declared Scott Diehl, PhD, co-author of the JAMA analysis, a genetics researcher at Rutgers School of Dental Medicine, and Professor and Principal Investigator at Rutgers Biomedical Health Sciences.
GenomeWeb notes in their latest coverage that with Orig3n’s purchase of Interleukin Genetics, Diehl is once again concerned that the genetic tests in question might find their way back to the market.
When GenomeWeb questioned Orig3n about the concerns surrounding Interleukin’s Ilustra product, a spokesperson stated, “that was simply before Orig3n’s time with the company and they do not have a part in it.” Blanchard added, “[We are] looking at the entire Interleukin portfolio and implementing the tests if and when we decide it is appropriate.”
Regardless of the decisions made by Orig3n on future genetic tests and genetic service offerings, coverage of this event highlights a myriad of concerns—from regulatory scrutiny to the pitfalls of acquiring existing diagnostic tests or laboratory assets—facing clinical laboratories, anatomic pathologists, and other medical professionals working in the ever-shifting landscape of the modern healthcare system.
—Jon Stone
Related Information:
This DNA Testing Company Is Violating Federal Lab Testing Rule
Orig3n Acquires Interleukin Genetics, a Genetics-based Personalized Health Company, to Advance the Future of Health Faster
Orig3n’s Purchase of Interleukin’s CLIA Lab May Appease CMS, But Some Question Plans for Test Assets
Biotech Company Offers Fitness and Beauty-Focused Genetic Tests
Interleukin Genetics Slashes 63 Percent of Workforce, Shuts down Program and Mulls Sale
‘DNA Day’ Planned for Ravens Game Undergoes Federal and State Scrutiny
Interleukin Shutting Down Genetic Testing Program, Lays Off Staff
Divergent Findings on Interleukin Gum Disease Risk Test Raise Questions about Clinical Use
Interleukin 1 Genetic Tests Provide No Support for Reduction of Preventive Dental Care
Controversial Gum Disease Risk Test Highlights Precision Medicine Marketing, Regulatory Issues
State and Federal Agencies Throw Yellow Flag Delaying Free Genetic Tests at NFL Games in Baltimore—Are Clinical Laboratories on Notice about Free Testing?
Jan 8, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Pathology, Laboratory Testing
Microbiome is once again leading scientists toward a new understanding of how human gut bacteria can impact the efficacy and side-effects of certain cancer therapies
Anatomic pathology researchers already know that a person’s genetics can affect the results of cancer treatments. Now it is becoming clear that a patient’s microbiome—which includes gut bacteria—may also impact the efficacy of particular cancer treatments. A recent study showed that gut bacteria can be used to determine whether a cancer drug will work for a certain individual and also if the patient might suffer side effects from certain cancer treatments.
Working with this knowledge, diagnostic test companies may possibly develop new clinical laboratory tests designed to help physicians better diagnose and treat cancer patients. This, in turn, advances personalized medicine and treatments for chronic diseases tailored to patients’ specific physiologies and conditions. This is a healthcare trend where medical laboratories can expect to play a critical role.
Gut Bacteria as Important as Genetics in Cancer Treatments
A recent article published in the journal Nature: npj Biofilms and Microbiomes, outlined a correlation between gut bacteria and side effects of irinotecan (sold as Camptosar), a drug used to treat metastatic colorectal cancer.
Libusha Kelly, PhD, Assistant Professor in the Departments of Systems and Computational Biology, and Microbiology and Immunology, led researchers from the Albert Einstein College of Medicine located in Bronx, N.Y., in conducting the study.
“We’ve known for some time that people’s genetic makeup can affect how they respond to a medication,” noted Kelly in an Einstein news release. “Now, it’s becoming clear that variations in one’s gut microbiome—the population of bacteria and other microbes that live in the digestive tract—can also influence the effects of treatment.”
Irinotecan is administered intravenously to colorectal cancer patients in an inactive form and is metabolized to an active form by liver enzymes. The drug is later converted back to an inactive form by other liver enzymes and the addition of a Glucuronidase chemical group. The irinotecan then enters the intestine for expulsion by the body.

Taken from the Einstein College of Medicine published study, the graph above illustrates “Two distinct metabolizer phenotypes or ‘metabotypes’ based on % SN-38 formation during a time course incubation of SN-38G with fecal samples from 20 individuals quantified by LC-MS/MS. Participants were sub-grouped into low (n = 16) and high (n = 4) metabolizer phenotypes. All samples were run in triplicate and values are the mean ± sem.” (Graphic copyright: Nature/Albert Einstein College of Medicine.)
However, bacteria residing in the digestive tract of some individuals prevent the medication from metabolizing properly and reactivates the medication, which transforms the irinotecan into a toxic substance that can cause side effects.
To perform the research, Kelly and her team collected fecal samples from 20 healthy individuals and treated those samples with inactive irinotecan. The samples were then examined and categorized by whether or not they were able to metabolize or reactivate the drug.
Identifying Potential for Side Effects in Patients a Powerful Tool for Medical Laboratories
Irinotecan can cause severe diarrhea and dehydration in up to 40% of patients who take the medication. By focusing on the presence of beta-glucuronidase (enzymes that are used to catalyze the breakdown of complex carbohydrates) the researchers found that gut bacteria can also be used to distinguish which patients will encounter side effects from the drug.
“As you can imagine, such patients are already quite ill, so giving them a treatment that causes intestinal problems can be very dangerous,” said Kelly in the news release. “At the same time, irinotecan is an important weapon against this type of cancer.”
Four of the 20 subjects in the study were determined to be high metabolizers. Due to differences in the composition of their microbiomes, the team concluded that the high metabolizers were more likely to experience side effects from irinotecan.
The research also demonstrated that beta-glucuronidase enzymes in the gut may adversely interact with some commonplace drugs, such as ibuprofen and other nonsteroidal anti-inflammatory medications (NSAIDs), morphine, and Tamoxifen, a drug that is prescribed mainly to breast cancer patients.
“In these cases, the issue for patients may not be diarrhea,” states Kelly in the news release. “Instead, if gut bacteria reactivate those drugs, then patients might be exposed to higher-than-intended doses. Our study provides a broad framework for understanding such drug-microbiome interactions.”
Microbiome Takes Center Stage in Pathology Research
As Dark Daily previously reported, from extending life to developing more powerful treatments for chronic diseases, the human microbiome is quickly becoming an important subject of research studies. The findings from such studies will trigger advances in precision medicine. And, the clinical laboratory assays developed from this research will give physicians the knowledge needed to select the most appropriate drug therapies and treatments for individual patients.
—JP Schlingman
Related Information:
Gut Bacteria Can Stop Cancer Drugs from Working
Gut Microbiome May Make Chemo Drug Toxic to Patients
Human Microbiome Signatures of Differential Colorectal Cancer Drug Metabolism
Researchers in Two Separate Studies Discover Gut Microbiome Can Affect Efficacy of Certain Cancer Drugs; Will Findings Lead to a New Clinical Laboratory Test?
Attention Microbiologists and Medical Laboratory Scientists: New Research Suggests an Organism’s Microbiome Might Be a Factor in Longer, More Active Lives
Mayo Clinic and Whole Biome Announce Collaboration to Research the Role of the Human Microbiome in Women’s Diseases Using Unique Medical Laboratory Tests
Jan 3, 2018 | Laboratory Instruments & Laboratory Equipment, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing
University of Turin study in Italy shows under-vacuum sealing systems reduce exposure to formaldehyde by 75% among nurses handling tissue biopsy specimens during surgery
Histology technicians and anatomic pathology (AP) laboratories regularly handle dangerous chemicals such as formaldehyde. They understand the risks exposure brings and take precautions to minimize those risks. However, in operating suites worldwide, nurses assisting surgeons also are being exposed to this nasty chemical.
Nurses must place biopsies and other tissues into buckets of formaldehyde to preserve the tissue between the operating room (OR) and histology laboratory. Formaldehyde, along with toluene, and xylene, is used to process and preserve biopsy tissue, displace water, and to create glass slides. It is an important substance that has long been used to maintain the viability of tissue specimens. Thus, exposure to formaldehyde among nurses is well-documented.
According to a National Academy of Sciences report, formalin, a tissue preservative that is a form of formaldehyde, has been linked to:
· Myeloid leukemia;
· Nasopharyngeal cancer; and,
· Sinonasal cancer.
However, as Dark Daily previously reported, “One alternative to storing specimens in buckets with formalin is to vacuum-seal specimens … [so] that both the quality management of the patient specimen and worker safety for handling the specimens are greatly improved.” (See Dark Daily, “Anatomic Pathology Labs Adopt New Ways to Package, Transport, and Store Specimens to Reduce Formalin and Improve Staff Safety in Operating Theaters and Histology Laboratories,” October 13, 2014.)
Now, motivated by increasing formaldehyde regulations in Europe, as well as the need to increase awareness of exposure risks, the University of Turin (Unito), and other hospitals in Italy’s Piedmont region, conducted a cross-sectional study of 94 female nurses who were being potentially exposed to formaldehyde.
Researchers Aim for “Formalin-Free” Hospitals
The Unito study showed that nurses using an under-vacuum sealing (UVS) system in ORs are exposed to levels of formaldehyde 75% lower than those who did not use the system. This study differs from other similar tests because the level of exposure is not just potential, due to environmental contamination, but confirmed with analytic data from specific urine analyses.
The researchers divided the nurses into two groups:
· One group immersed samples in containers of formaldehyde following standard procedures;
· The other group worked in operating rooms equipped with a UVS system.
The researchers described a UVS system that called for the tissue removed during surgery to be sealed in a medical grade vacuum bag and refrigerated at four degrees centigrade before being transferred to the lab for fixation.
One example of a UVS system is TissueSAFE plus, developed by Milestone Medical, located in Bergamo, Italy, and Kalamazoo, Mich. According to the company’s website, the system, “Eliminates formalin in the operating theatre and allows a controlled formalin-free transfer of biospecimens to the laboratory.”

The image above is from a research paper by Richard J. Zarbo, MD, Pathology and Laboratory Medicine, Henry Ford Health System. It describes “five validation trials of new vacuum sealing technologies that change the approach to the preanalytic ‘front end’ of specimen transport, handling, and processing, and illustrate their adaptation and integration into existing Lean laboratory operations with reduction in formalin use and personnel exposure to this toxic and potentially carcinogenic fixative.” (Image copyright: Henry Ford Health System/Springer International Publishing.)
Increased Scrutiny Leads to New Pathology Guidelines
In a paper published in Toxicology Research, a journal of The Royal Society of Chemistry, the researchers noted a marked difference related to the adoption of the under-vacuum sealing procedure, as an alternative to formaldehyde for preserving tissues. “Nurses, operating in surgical theatres, are traditionally exposed to formaldehyde because of the common and traditional practice of immersing surgical samples, of a size ranging between two and 30 centimeters, in this preservative liquid (three to five liters at a time) to be later transferred to a [histopathology] lab,” the authors wrote. “We evaluated the conditions favoring the risk of exposure to this toxic reagent and the effect of measures to prevent it.”
Throughout Europe, increased scrutiny has forced medical pathology associations to write new guidelines that accept alternative methods to formaldehyde-based tissue preservation methods.
“In Europe, and in Italy in particular, the level of attention to formaldehyde exposure in the public health hospital system has become very high, forcing pathology associations to rewrite guidelines,” Marco Bellini, General Manager of the Medical Division at Milestone Medical, told Dark Daily. “What makes this study unique from many other similar tests is that the level of exposure has been confirmed with data from specific urine analyses,” he added.
The Italian Society of Pathological Anatomy and Diagnostic Cytology (SIAPEC), a division of the International Academy of Pathology, wrote general guidelines for AP labs that have been accepted and officially published by the Italian Ministry of Health.
The main topic of these guidelines is the preanalytical aspects of specimen collection, transportation, and preservation, where the vacuum method has been indicated as a valid alternative to improve the standardization of these crucial steps in pathology. By moving the starting point for specimen fixation from the OR to the histology labs, parameters can be controlled and documented, with the main advantage of reducing formaldehyde exposure by operators at the collection point.
These guidelines will be presented at the European Society of Pathology (ESP) with the intent to extending them throughout Europe.
Toluene’s and Xylene’s Effects Studied
Formaldehyde is not the only potentially harmful substance in the clinical laboratory. As previously noted, common solvents toluene and xylene also are potentially hazardous.
In fact, a study of pathologists, lab technicians, and scientists who work with toluene and xylene published in the Journal of Rheumatology found that the chance of acquiring Raynaud Syndrome (a vascular condition) doubled for those workers. (See Dark Daily, “Health of Pathology Laboratory Technicians at Risk from Common Solvents like Xylene and Toluene,” July 5, 2011.)
Medical laboratory leaders are reminded to initiate processes that ensure safe specimen handling, transport, and processing, as well as workflow changes that eliminate chemical odors in the lab. Studies, such as those cited above, may provide information necessary to affect change.
—Donna Marie Pocius
Related Information:
Formaldehyde Fact Sheet
Towards a Formalin-Free Hospital: Levels of 15-F2t-isoprostane and malondialdehyde to Monitor Exposure to Formaldehyde in Nurses from Operating Theatres
Histologic Validation of Vacuum Sealed, Formalin-Free Tissue Preservation, and Transport System
Notes Regarding the Use of Formalin, Reclassified as “Carcinogenic”
Formaldehyde Substitute Fixatives: Analysis of Macroscopy, Morpholologic Analysis, and Immunohistochemical Analysis
Anatomic Pathology Labs Adopt New Ways to Package, Transport, and Store Specimens to Reduce Formalin and Improve Staff Safety in Operating Theaters and Histology Laboratories
Health of Pathology and Laboratory Technicians at Risk from Common Solvents Like Xylene and Toluene
National Academy of Sciences Confirms that Formaldehyde Can Cause Cancer in a Finding that has Implications for Anatomic Pathology and Histology Laboratories
Dec 29, 2017 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory News, Laboratory Pathology, Laboratory Testing
If the link between certain types of gut bacteria and improved effectiveness of certain cancer treatments can be leveraged, then medical laboratories could soon have another diagnostic tool to use in supporting physicians with cancer care
From improving treatments for chronic diseases to extending lives, gut microbiome (bacteria that is part of human microbiota) has been at the forefront of developing clinical laboratory testing and anatomic pathology diagnostic technologies in recent years. Now, two studies recently published in the online journal Science confirm research that the “composition” of gut bacteria may have a significant influence on the effectiveness of certain cancer drugs.
The goal of both studies was to determine whether there was a link between gut bacteria and the efficacy of cancer drugs known as PD-1 inhibitors. These drugs are used for several types of cancer, including:
- Melanoma;
- Lung;
- Bladder; and,
- Stomach cancers.
They function by freeing up the immune system to attack cancer cells.
Greater Bacterial Diversity in Gut Brings Improved Response to PD-1 Inhibitors
One of the studies, “Gut Microbiome Modulates Response to Anti–PD-1 Immunotherapy in Melanoma Patients,” found that a microbiome populated with “good” bacteria can elevate the potency of certain drug treatments. The researchers discovered that the gut bacteria in patients who responded well to PD-1 inhibitors differed from that found in patients who did not respond to the treatment.
For this study, researchers at the MD Anderson Cancer Center at the University of Texas collected oral, gut, and fecal microbiome samples and tumor biopsies from 112 patients with advanced melanoma. Clinical laboratorians took the samples before and after PD-1 treatments. They divided the patients into two groups—responders and non-responders—and profiled each microbiome using genetic sequencing.
“What we found was impressive: There were major differences both in the diversity and composition of the gut microbiome in responders versus non-responders,” Jennifer Wargo, MD, MMSc, leader of the study, told STAT. “Those who did well had greater bacterial diversity in their gut, whereas those whose tumors didn’t much shrink had fewer varieties of microbes present.”
Melanoma patients who experienced success with PD-1 therapy had a more diverse microbiome and higher concentrations of bacteria known as Ruminococcus and Faecalibacterium. Patients involved in the study who did not respond well to PD-1 therapy had the presence of another bacterium called Bacteroidales.

Jennifer Wargo, MD (above center) with her team at the MD Anderson laboratories. The researchers cautioned that clinical trials are needed before a definitive conclusion can be reached on whether altering gut bacteria can improve the effectiveness of PD-1 therapy. “If you’re changing the microbiome, depending on how you do it, it may not help you—and it might harm you,” Wargo emphasized in STAT. “Don’t try this at home.” (Photo copyright: MD Anderson.)
Antibiotics Can Reduce Effectiveness of PD-1Therapy
The other study, “Gut Microbiome Influences Efficacy of PD-1-based Immunotherapy Against Epithelial Tumors,” discovered that some drug therapies were less effective in patients who were also taking antibiotics to treat infections shortly before beginning treatment with PD-1 drugs.
Researchers for this study, led by Laurence Zitvogel, MD, PhD, of the Gustave Roussy Cancer Campus in Villejuif, France, examined 249 patients who were given a PD-1 inhibitor for lung, kidney, or urinary tract cancers. A little over one fourth of these patients had recently taken antibiotics, which can strip the gut of essential bacteria necessary to treat infections.
The team found that patients who had ingested an antibiotic relapsed faster and did not live as long as patients who had not taken an antibiotic before receiving PD-1 therapy. When they analyzed variances between patients who responded well to treatment versus patients who did not, they detected the presence of Akkermansia muciniphila, a mucin-degrading bacterium, in the responders.
Personalized Treatment Based on Each Patient’s Gut Microbiome
The culmination of this type of research raises questions about how cancer medications may interact with microbiomes.
“Should we be profiling the gut microbiome in cancer patients going into immunotherapy?” asked Wargo in the STAT article. “And, should we also be limiting, or closely monitoring, the antibiotic use in these patients?
“This is all very context-specific, and multiple different factors need to be considered on how best to change the microbiome,” she continued. “When it comes to optimizing cancer therapy, treatments will have to be heavily personalized, based on what a patient’s gut microbiome looks like already.”
Diagnostic tests that could determine whether a certain drug will be beneficial for a patient would perform a critical role in healthcare decision-making. Since cancer drug treatments can cost tens of thousands of dollars or more, it would be advantageous to know which therapies would be optimal for individual patients. The hope is that in the future, clinicians, working with anatomic pathologists and clinical laboratories, will have the tools needed to ascertain if patient’s microbiomes will best work with a particular drug and if they would likely encounter any side effects.
—JP Schlingman
Related Information:
Patients’ Gut Bugs May Play Role in Cancer Care
Gut Microbiome Modulates Response to Anti–PD-1 Immunotherapy in Melanoma Patients
Gut Microbiome Influences Efficacy Of PD-1–Based Immunotherapy Against Epithelial Tumors
Your Gut Bacteria Could Determine How You Respond to Cutting-edge Cancer Drugs
The Bacteria in Your Gut Could Help Determine if a Cancer Therapy Will Work
Attention Microbiologists and Medical Laboratory Scientists: New Research Suggests an Organism’s Microbiome Might Be a Factor in Longer, More Active Lives
Get the Poop on Organisms Living in Your Gut with a New Consumer Laboratory Test Offered by American Gut and uBiome
Mayo Clinic and Whole Biome Announce Collaboration to Research the Role of the Human Microbiome in Women’s Diseases Using Unique Medical Laboratory Tests










