Nov 14, 2018 | Coding, Billing, and Collections, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
Physicians in Saskatchewan called for changes after wait times for anatomic pathology test results reached six weeks or more
Anatomic pathologist and histopathologist shortages have plagued the single-payer healthcare systems in Canada and the United Kingdom (UK) in recent years. The consequence is increased wait times for physicians in both countries to receive medical laboratory test results, which increases wait times across the entire healthcare continuum.
However, one Canadian province significantly reduced a backlog that had pushed wait times for surgical pathology test results to six weeks or more. It did this by having its pathologists perform first-stage examinations normally completed by pathology assistants or medical technologists.
The Saskatchewan Health Authority (SHA) announced in October it had cleared nearly half of the 2,600-plus biopsies that were waiting to be processed at hospital labs in Regina and Saskatoon, the Regina Leader-Post reported.
“I think we’ve been making amazing progress in the work,” Lenore Howey, Executive Director of Laboratory Services at SHA, told the newspaper. “It’s always good to take time to know and understand your process, so that we can put the right resources in the right places.”
Getting Anatomic Pathologists Involved
Howey stated the SHA cleared cases by having pathologists “assist with the work in the first phase”—or gross examination stage—of a biopsy. This is the part of the process during which pathology assistants or medical laboratory technologists typically record the size, weight, and description of a specimen and look for pathological changes.
In addition, the SHA hired an additional pathologist assistant and three histology/cytology technologists—one on a permanent basis and two on a temporary basis. Other improvements include:
- Working toward resolving problems with voice recognition transcription software being piloted in Regina for the gross examination phase of processing; and;
- Implementing an electronic specimen tracking system in Saskatoon, which eventually also may be used in Regina.
Physicians Express Dissatisfaction with Wait Times
Physicians attending the Saskatchewan Medical Association’s Spring Representative Assembly in May raised the backlog issue with Health Minister Jim Reiter, complaining about the impact on patient care. At that point, the backlog of pathology cases had hit 1,662 in Regina, while Saskatoon’s caseload totaled 1,005. Many of these biopsies involve cancer patients, thus delaying a diagnosis and the start of an appropriate treatment for these patients.
“I’m trying to get things done as expeditiously as possible,” urologist Francisco Garcia, MD, told the Leader-Post, “but for the first five or six weeks, I’m handcuffed in terms of what I’m able to do.”
Now, thanks to SHA’s efforts, as of Oct. 2 specimens in progress dropped to 785 in Regina and 748 in Saskatoon. Both numbers are within range of SHA’s target of 750.

“We do not have a backlog right now,” Lenore Howey, Executive Director of Laboratory Services at SHA, told the Leader-Post. “Our system is very stable, but we do have checks and balances to put in place so that we would never get there again, which we didn’t have prior.” (Photo copyright: Saskatchewan Health Authority.)
Wait Times Impacting Patient Care Worldwide
While Saskatchewan appears to have solved its most recent pathology reporting issue, this is not the first time the province has dealt with delays in lab testing reports. In 2011, Dark Daily reported on lengthy turnaround times for anatomic pathology test reports that averaged more than 12 days, which was blamed on shortage of pathologists dating back to 2001. (See, “Pathologist Shortage and Delays in Lab Test Reports Get Publicity in Saskatchewan,” August 15, 2011.)
And in October, Dark Daily reported that cancer patients in the UK are experiencing record waiting times for treatments, with more than 3,000 people waiting longer than two months to begin care, iNews reported. Delays there are being blamed in part on severe shortages of pathology staff. A 2017 workforce survey by the Royal College of Pathologists reported that only 3% of the National Health Service (NHS) histopathology departments responding to the survey had adequate staff. (See, “Shortage of Histopathologists in the United Kingdom Now Contributing to Record-Long Cancer-Treatment Waiting Times in England,” October 31, 2018.)
“Making sure pathology services can cope with current and future demand is essential if we are to ensure early diagnosis and improve outcomes for patients,” Jo Martin, PhD, President of the Royal College of Pathologists, told the BBC.
Increased workloads due to new NHS screening programs and an approaching retirement crisis—a quarter of all histopathologists in the UK are aged 55 or over—has caused the Royal College of Pathologists to call for more funded training places, better IT systems, and further investment in pathology services.
While the US healthcare system is not currently experiencing a shortage of clinical laboratory staff or anatomic pathologists, shortages in other countries illustrate the impact any delay in reporting results can have on patient care.
—Andrea Downing Peck
Related Information:
Backlog of Pathology Tests Cleared in Province
Technology and Staff Shortages Contribute to Biopsy Backlog
Pathology Staff Shortages Causing Delays to Cancer Diagnosis, Says Report
Cancer Waiting Times at their Worst Ever Level
Histopathology Workforce Survey 2018
Pathologists Shortage ‘Delaying Cancer Diagnosis’
Pathologists Shortage and Delays in Lab Test Reports Get Publicity in Saskatchewan
Shortage of Histopathologists in the United Kingdom Now Contributing to Record-Long Cancer-Treatment Waiting Times in England
Nov 5, 2018 | Digital Pathology, Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing
Computer-assisted analysis using Google’s LYNA algorithm shows significant gains in speed required to analyze stained lymph node slides and sensitivity of micrometastases detection in two recent studies
Anatomic pathologists understand the complexities of reviewing slides and samples for signs of cancer’s spread. Two studies involving a new artificial intelligence (AI) algorithm from Google (NASDAQ:GOOGL) claim their “deep learning” LYmph Node Assistant (LYNA) provides increases to both the speed at which pathologists can analyze slides and improved accuracy in detecting metastatic breast cancer within the slide samples used for the studies.
Google’s first study was published in the Archives of Pathology and Laboratory Medicine and investigated the accuracy of the algorithm using digital pathology slides. Google’s second study, published in The American Journal of Surgical Pathology, looked at how pathologists might harness the algorithm to improve workflows and use the tool in a clinical setting.
Medical laboratories and other diagnostics providers are already familiar with the improvement potential of automation and other technology-based approaches to diagnosis and analysis. Google’s LYNA is an example of how AI and machine learning improvements can serve as a supplement to—not a replacement for—the skills of experts at pathology groups and clinical laboratories.
Early research done by Google indicates that integrating LYNA into existing workflows could allow pathologists to spend less time analyzing slides for minute details. Instead, they could focus on other more challenging tasks while the AI analyzes gigapixels worth of slide data to highlight regions of concern in slides and samples for deeper manual inspection.
LYNA Achieves 99% Accuracy in Study of Metastatic Breast Cancer Detection
According to the research cited in a Google AI Blog post, roughly 25% of metastatic lymph node staging classifications would change if subjected to a second pathologic review. They further note that when faced with time constraints, detection sensitivity for small metastases on individual slides can be as low as 38%.
In findings published in Archives of Pathology and Laboratory Medicine, Google researchers analyzed whole slide images from hematoxylin-eosin-stained lymph nodes for 399 patients sourced from the Camelyon16 challenge dataset. Of those slides, researchers used 270 to train LYNA and the remaining 129 for analysis. They then compared the LYNA findings to those of an independent lab using a different scanner.
“LYNA achieved a slide-level area under the receiver operating characteristic (AUC) of 99% and a tumor-level sensitivity of 91% at one false positive per patient on the Camelyon16 evaluation dataset,” the researchers stated. “We also identified [two] ‘normal’ slides that contained micrometastases.”
Google’s algorithm later received an AUC of 99.6% on a secondary dataset.
“Artificial intelligence algorithms can exhaustively evaluate every tissue patch on a slide, achieving higher tumor-level sensitivity than, and comparable slide-level performance to, pathologists,” the researchers continued. “These techniques may improve the pathologist’s productivity and reduce the number of false negatives associated with morphologic detection of tumor cells.”
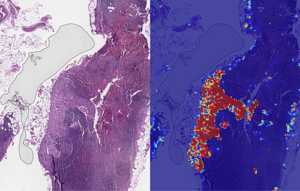
Left: sample view of a slide containing lymph nodes, with multiple artifacts: the dark zone on the left is an air bubble, the white streaks are cutting artifacts, the red hue across some regions are hemorrhagic (containing blood), the tissue is necrotic (decaying), and the processing quality was poor. Right: LYNA identifies the tumor region in the center (red), and correctly classifies the surrounding artifact-laden regions as non-tumor (blue). (Image and caption copyright: Google AI Blog.)
Faster Analysis through Software Assistance
Rapid diagnosis helps improve cancer outcomes. Yet, manually reviewing and analyzing complex digital slides is time-consuming. Time constraints might also lead to false negatives due to micrometastases or small suspicious regions that slip by pathologists undetected.
The Google research team of the study published in The American Journal of Surgical Pathology sought to gauge the impact LYNA might have on the histopathologic review of lymph nodes for trained pathologists. In their multi-reader multi-case study, researchers analyzed differences in both sensitivity of detecting micrometastases and the average review time per image using both computer-aided detection and unassisted detection for six pathologists across 70 slides.
Using the LYNA algorithm to identify and outline regions likely to contain tumors, the researchers found that sensitivity increased from 83% to 91%. The time to review slides also saw a significant reduction from 116 seconds in the unassisted mode to 61 seconds in the assisted mode—a time savings of roughly 47%.
“Although some pathologists in the unassisted mode were less sensitive than LYNA,” the researchers stated, “all pathologists performed better than the algorithm alone in regard to both sensitivity and specificity when reviewing images with assistance.”
The Future of Digital Pathology using LYNA
While the two studies show positive results, both studies also reveal shortcomings. Google highlighted both limited dataset sizes and simulated diagnostic workflows as potential concerns and areas on which to focus future studies.
Still, Google’s researchers believe that algorithms such as LYNA will help to power the future of diagnostics as healthcare in the digital era continues to mature. “We remain optimistic,” state the authors of the Google AI Blog post, “that carefully validated deep learning technologies and well-designed clinical tools can help improve both the accuracy and availability of pathologic diagnosis around the world.”
While other industries see risk in the growth of AI, both studies performed by researchers at Google show how computer-assisted workflows and machine learning could accentuate and bolster the skills of trained diagnosticians, such as anatomic pathologists and clinical laboratory technicians. By working to compensate for weak points in both human skill and computer reasoning, the outcome could be greater than either AI or humans can achieve separately.
—Jon Stone
Related Information:
Google Creates AI to Detect When Breast Cancer Spreads
Google Deep Learning Tool 99% Accurate at Breast Cancer Detection
Google’s AI Software Seeks to Detect Advanced Breast Cancer Better Than We Have Before
Google’s AI Is Better at Spotting Advanced Breast Cancer than Pathologists
Google AI Claims 99% Accuracy in Metastatic Breast Cancer Detection
Applying Deep Learning to Metastatic Breast Cancer Detection
Assisting Pathologists in Detecting Cancer with Deep Learning
Diagnostic Assessment of Deep Learning Algorithms for Detection of Lymph Node Metastases in Women with Breast Cancer
Nov 2, 2018 | Coding, Billing, and Collections, Compliance, Legal, and Malpractice, Digital Pathology, Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
Patient privacy, ethics of monetizing not-for-profit data, and questions surrounding industry conflicts appear after the public announcement of an arrangement to grant exclusive access to academic pathology slides and samples
Clinical laboratories and anatomic pathology groups already serve as gatekeepers for a range of medical data used in patient treatments. Glass slides, paraffin-embedded tissue specimens, pathology reports, and autopsy records hold immense value to researchers. The challenge has been how pathologists (and others) in a not-for-profit academic center could set themselves up to potentially profit from their exclusive access to this archived pathology material.
Now, a recent partnership between Memorial Sloan Kettering Cancer Center (MSK) and Paige.AI (a developer of artificial intelligence for pathology) shows how academic pathology laboratories might accomplish this goal and serve a similar gatekeeper role in research and development using the decades of cases in their archives.
The arrangement, however, is not without controversy.
New York Times, ProPublica Report
Following an investigative report from the New York Times (NYT) and ProPublica, pathologists and board members at MSK are under fire from doctors and scientists there who have concerns surrounding ethics, exclusivity, and profiting from data generated by physicians and but owned by MSK.
“Hospital pathologists have strongly objected to the Paige.AI deal, saying it is unfair that the founders received equity stakes in a company that relies on the pathologists’ expertise and work amassed over 60 years. They also questioned the use of patients’ data—even if it is anonymous—without their knowledge in a profit-driven venture,” the NYT article states.
Prominent members of MSK are facing scrutiny from the media and peers—with some relinquishing stakes in Paige.AI—as part of the backlash of the report. This is an example of the perils and PR concerns lab stakeholders might face concerning the safety of data sharing and profits made by medical laboratories and other diagnostics providers using patient data.
Controversy Surrounds Formation of Paige.AI/MSK Partnership
In February 2018, Paige.AI announced closing the deal on a $25-million round of Series A funding, and in gaining exclusive access to 25-million pathology slides and computational pathology intellectual property held by the Department of Pathology at Memorial Sloan Kettering. Coverage by TechCrunch noted that while MSK received an equity stake as part of the licensing agreement, they were not a cash investor.
Creation of the company involved three hospital insiders and three additional board members with the hospital itself established as part owner, according to STAT.
Unnamed officials told the NYT that board members at MSK only invested in Paige.AI after earlier efforts to generate outside interest and investors were unsuccessful. NYT’s coverage also notes experts in non-profit law and corporate governance have raised questions as to compliance with federal and state laws that govern nonprofits in light of the Paige.AI deal.
Growing Privacy Fallout and Potential Pitfalls for Medical Labs
The original September 2018 NYT coverage noted that Klimstra intends to divest his ownership stake in Paige.AI. Later coverage by NYT in October, notes that Democrat Representative Debbie Dingell of Michigan submitted a letter questioning details about patient privacy related to Paige.AI’s access to MSK’s academic pathology resources.
Privacy continues to be a focus for both media and regulatory scrutiny as patient data continues to fill electronic health record (EHR) systems as well as research and commercial databases. Dark Daily recently covered how University of Melbourne researchers demonstrated how easily malicious parties might reidentify deidentified data. (See “Researchers Easily Reidentify Deidentified Patient Records with 95% Accuracy; Privacy Protection of Patient Test Records a Concern for Clinical Laboratories”, October 10, 2018.)
According to the NYT, MSK also issued a memo to employees announcing new restrictions on interactions with for-profit companies with a moratorium on board members investing in or holding board positions in startups created within MSK. The nonprofit further noted it is considering barring hospital executives from receiving compensation for their work on outside boards.
However, MSK told the NYT this only applies to new deals and will not affect the exclusive deal between Paige.AI and MSK.
“We have determined,” MSK wrote, “that when profits emerge through the monetization of our research, financial payments to MSK-designated board members should be used for the benefit of the institution.”
There are no current official legal filings regarding actions against the partnership. Despite this, the arrangement—and the subsequent fallout after the public announcement of the arrangement—serve as an example of pitfalls medical laboratories and other medical service centers considering similar arrangements might face in terms of public relations and employee scrutiny.
Risk versus Reward of Monetizing Pathology Data
While the Paige.AI situation is only one of multiple concerns now facing healthcare teams and board members at MSK, the events are an example of risks pathologists take when playing a role in a commercial enterprise outside their own operations or departments.
In doing so, the pathologists investing in and shaping the deal with Paige.AI brought criticism from reputable sources and negative exposure in major media outlets for their enterprise, themselves, and MSK as a whole. The lesson from this episode is that pathologists should tread carefully when entertaining offers to access the patient materials and data archived by their respective anatomic pathology and clinical laboratory organizations.
—Jon Stone
Related Information:
Sloan Kettering’s Cozy Deal with Start-Up Ignites a New Uproar
Paige.AI Nabs $25M, Inks IP Deal with Sloan Kettering to Bring Machine Learning to Cancer Pathology
Sloan Kettering Executive Turns Over Windfall Stake in Biotech Start-Up
Cancer Center’s Board Chairman Faults Top Doctor over ‘Crossed Lines’
Memorial Sloan Kettering, You’ve Betrayed My Trust
LVHN Patient Data Not Shared with For-Profit Company in Sloan Kettering Trials
Researchers Easily Reidentify Deidentified Patient Records with 95% Accuracy; Privacy Protection of Patient Test Records a Concern for Clinical Laboratories
Oct 31, 2018 | Laboratory Hiring & Human Resources, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing
Only 3% of histopathology departments that responded to the Royal College of Pathologists’ workforce census reported enough staff to meet clinical demand
There is a chronic shortage of histopathologists in the United Kingdom (UK) and it is being blamed for cancer treatment waiting times that now reach the worst-ever levels, as National Health Service (NHS) training initiatives and other steps fail to keep pace with growing demand for diagnostic services.
For US anatomic pathologists and clinical laboratory managers, headlines from the UK reveal the impact a shortage of trained histopathologists (UK’s version of anatomic pathologists) and support technical staff can have on patient care when longer wait times for pathology support and diagnosis become the norm.
Royal College of Pathologists Report
The extent of the UK-wide histopathology staff shortages was highlighted in a recently released 2017 workforce census by the Royal College of Pathologists (RCPath). Of the 103 histopathology departments that responded to a survey, only 3% said they had enough staff to meet the current clinical demand! And 45% of departments had to outsource work, while half of the departments were forced to use more expensive temporary workers.
“The cost of staff shortages across histopathology departments is high for both patients and for our health services,” Jo Martin, PhD, President of the Royal College of Pathologists, noted in a statement announcing the survey results. “For patients, it means worrying delays in diagnosis and treatment. For NHS hospitals, it means spending more resources on [temporary] doctors to fill staffing gaps, or outsourcing services. We estimate this cost [to be] £27 million ($35.2 million) each year across the UK health service—money that could be better invested in staff and new diagnostic equipment.”

Royal College of Pathologists President Jo Martin, PhD, is calling on the National Health Service to take additional steps to increase the number of pathologists working in the United Kingdom, including more funded training places. That’s following the release of a Royal College of Pathologists workforce survey, which reported only 3% of histopathology departments in the UK have enough staff to meet clinical demand. (Photo copyright: Twitter.)
According to iNews, NHS England recorded its worst cancer treatment waiting times on record in July 2018, with more than 3,000 people waiting longer than two months for cancer treatment to begin. NHS’ target is for 85% of patients to begin cancer treatment within 62 days of being referred by their general practitioner.
Anatomic pathologists in the United States should consider how the UK’s average delay in starting cancer treatment affects patients in that country. It is a metric that patients in the US would not tolerate. However, in the UK’s single payer system, the government’s National Health Service sets the budgets for training new physicians, including histopathologists. For many years, the pathology profession in the UK has regularly advocated for increasing the number of histopathologists trained each year by the medical schools in that country.
In July, the number of patients waiting for treatment longer than 60 days fell to 78.2%, the 31st month in a row the target was breached, iNews reported.
“We know that histopathology consultant shortages contribute to at least part of that problem,” Martin told iNews.
The RCPath report highlights the intense pressures on histopathologists—most of whom working in understaffed laboratories—face from increased workloads, as new NHS cancer screening initiatives, an aging population, and a shift toward precision medicine result in a rising number of cases being referred to labs.
“Demand for pathology services has grown significantly in recent years and continues to grow,” Martin noted in the RCPath statement. “The pathology workforce has not increased in line with this demand. If this trend continues unchecked, clinical services could be in jeopardy. Making sure pathology services can cope with current and future demand is essential if we are to ensure early diagnosis and improve outcomes for patients.”
Lack/Loss of Trained Histopathologists an Ongoing Problem
This is not the first time the alarm has been sounded in the UK over the lack of investment in trained pathologists along with a growing shortage of trained professional staff. In 2017, Dark Daily reported on calls by pathologists and other physicians for the UK government to increase funding for trained medical laboratory professionals to avert a predicted critical shortage in laboratory staffing within the next decade. (See “Pathologists and Physicians in United Kingdom Comment on How Shortage of Medical Laboratory Professionals Could Soon Delay Essential Diagnostic, Therapeutic Testing,” February 6, 2017.)
In its most recent workforce report, The Royal College of Pathologists is reiterating its call for:
- Increased funding for training;
- Better information technology (IT) for day-to-day work;
- Capital investment to implement digital pathology more widely; and,
- Development of advanced clinical practitioner apprenticeships to help more biomedical scientists become independent practitioners who would work alongside medically-qualified histopathologists.
Long-term, the organization is calling for additional training slots for pathologists in universities as well as other changes to draw more scientists into the field.
Aging Pathology Staff Means Shortages in US a Possibility
The NHS has stopped short of declaring the pathologist shortage a crisis. Instead, a Department of Health and Social Care spokesperson in an interview with the BBC highlighted recent initiatives taken in response to the shortage. “There are hundreds more pathologists in the NHS compared to 2010 and hospitals have reduced spending on temporary agency staff by over half a billion pounds in the last year,” the spokesperson noted. “We are listening to staff, encouraging more flexible working, and have increased medical training places for home-grown doctors by 25%, to ensure the NHS has the workforce it needs for the future.”
Despite those steps, the NHS may have more work to do. According to the Royal College of Pathologists workplace survey, a quarter of all histopathologists in the UK are 55 or older, adding an approaching retirement crisis to the existing shortage.
US anatomic pathology groups and clinical laboratories should monitor these events with a keen eye. The American pathology industry is challenged by many of the same trends, such as an aging workforce and lackluster replacement initiatives. Time will tell if the US learns from the UK’s experience.
—Andrea Downing Peck
Related Information:
College Report Finds Severe Staff Shortages Across Services Vital to Cancer Diagnosis
Pathologist Shortage ‘Delaying Cancer Diagnosis’
Cancer Treatment Waiting Times Worsening Amid Shortage of Pathology Staff
Histopathology Staff Shortages ‘Affecting Cancer Diagnoses’
Cancer Waiting Times at Their Worst Ever Level
Meeting Pathology Demand
Pathology Staff Shortages Causing Delays to Cancer Diagnosis, Says Report
Pathologists and Physicians in United Kingdom Comment on How Shortage of Medical Laboratory Professionals Could Soon Delay Essential Diagnostic, Therapeutic Testing
Oct 26, 2018 | Digital Pathology, Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing
Future EHRs will focus on efficiency, machine learning, and cloud services—improving how physicians and medical laboratories interact with the systems to support precision medicine and streamlined workflows
When the next generation of electronic health record (EHR) systems reaches the market, they will have advanced features that include cloud-based services and the ability to collect data from and communicate with patients using mobile devices. These new developments will provide clinical laboratories and anatomic pathology groups with new opportunities to create value with their lab testing services.
Proposed Improvements and Key Trends
Experts with EHR developers Epic Systems, Allscripts, Accenture, and drchrono spoke recently with Healthcare IT News about future platform initiatives and trends they feel will shape their next generation of EHR offerings.
They include:
- Automation analytics and human-centered designs for increased efficiency and to help reduce physician burnout;
- Improved feature parity across mobile and computer EHR interfaces to provide patients, physicians, and medical laboratories with access to information across a range of technologies and locations;
- Integration of machine learning and predictive modeling to improve analytics and allow for better implementation of genomics-informed medicine and population health features; and
- A shift toward cloud-hosted EHR solutions with support for application programming interfaces (APIs) designed for specific healthcare facilities that reduce IT overhead and make EHR systems accessible to smaller practices and facilities.
Should these proposals move forward, future generations of EHR platforms could transform from simple data storage/retrieval systems into critical tools physicians and medical laboratories use to facilitate communications and support decision-making in real time.
And, cloud-based EHRs with access to clinical labs’ APIs could enable those laboratories to communicate with and receive data from EHR systems with greater efficiency. This would eliminate yet another bottleneck in the decision-making process, and help laboratories increase volumes and margins through reduced documentation and data management overhead.
Cloud-based EHRs and Potential Pitfalls
Cloud-based EHRs rely on cloud computing, where IT resources are shared among multiple entities over the Internet. Such EHRs are highly scalable and allow end users to save money by hiring third-party IT services, rather than maintaining expensive IT staff.
Kipp Webb, MD, provider practice lead and Chief Clinical Innovation Officer at Accenture told Healthcare IT News that several EHR vendors are only a few years out on releasing cloud-based inpatient/outpatient EHR systems capable of meeting the needs of full-service medical centers.
While such a system would mean existing health networks would not need private infrastructure and dedicate IT teams to manage EHR system operations, a major shift in how next-gen systems are deployed and maintained could lead to potential interoperability and data transmission concerns. At least in the short term.
Yet, the transition also could lead to improved flexibility and connectivity between health networks and data providers—such as clinical laboratories and pathologist groups. This would be achieved through application programming interfaces (APIs) that enable computer systems to talk to each other and exchange data much more efficiently.
“Perhaps one of the biggest ways having a fully cloud-based EHR will change the way we as an industry operate will be enabled API access.” Daniel Kivatinos, COO and founder of drchrono, told Healthcare IT News. “You will be able to add other partners into the mix that just weren’t available before when you have a local EHR install only.”

Paul Black, CEO of Allscripts, believes these changes will likely require more than upgrading existing software or hardware. “The industry needs an entirely new approach to the EHR,” he told Healthcare IT News. “We’re seeing a huge need for the EHR to be mobile, cloud-based, and comprehensive to streamline workflow and get smarter with every use.” (Photo copyright: Allscripts.)
Reducing Physician Burnout through Human-Centered Design
As Dark Daily reported last year, EHRs have been identified as contributing to physician burnout, increased dissatisfaction, and decreased face-to-face interactions with patients.
Combined with the increased automation, Carl Dvorak, President of Epic Systems, notes next-gen EHR changes hold the potential to streamline the communication of orders, laboratory testing data, and information relevant to patient care. They could help physicians reach treatment decisions faster and provide laboratories with more insight, so they can suggest appropriate testing pathways for each episode of care.
“[Automation analytics] holds the key to unlocking some of the secrets to physician well-being,” Dvorak told Healthcare IT News. “For example, we can avoid work being unnecessarily diverted to physicians when it could be better managed by others.”
Black echoes similar benefits, saying, “We believe using human-centered design will transform the way physicians experience and interact with technology, as well as improve provider wellness.”
Some might question the success of the first wave of EHR systems. Though primarily built to address healthcare reform requirements, these systems provided critical feedback and data to EHR developers focused not on simply fulfilling regulatory requirements, but on meeting the needs of patients and care providers as well.
If these next-generations systems can help improve the quality of data recording, storage, and transmission, while also reducing physician burnout, they will have come a long way from the early EHRs. For medical laboratory professionals, these changes will likely impact how orders are received and lab results are reported back to doctors in the future. Thus, it’s worth monitoring these developments.
—Jon Stone
Related Information:
Next-Gen EHRs: Epic, Allscripts and Others Reveal Future of Electronic Health Records
Next-Gen IT Infrastructure: A Nervous System Backed by Analytics and Context
EHR Systems Continue to Cause Burnout, Physician Dissatisfaction, and Decreased Face-to-Face Patient Care
Oct 19, 2018 | Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Managed Care Contracts & Payer Reimbursement, Management & Operations
Employers and consumers continue to pay more for health benefits from one year to the next, continuing a trend that is not auspicious for clinical laboratories and anatomic pathology groups
Most clinical laboratories don’t have the capability to collect payments from patients at time of service the same way patients pay doctors during office visits. Thus, Milliman’s annual report which details the increasing amounts patients are expected to pay out of their own pockets should be of interest to clinical laboratory managers and stakeholders. As this trend accelerates, labs will need to adopt new procedures and technologies to conduct business and remain profitable.
The Milliman Medical Index report (MMI) details how much consumers are predicted to pay for healthcare each year, as compared to previous years. Milliman, a Seattle-based independent actuarial and consulting firm with offices throughout the world, examines healthcare costs, property and casualty insurance, life insurance, financial services, and employee benefits.
Milliman released its first MMI in 2005. That year, the average annual medical cost for a family of four was $12,214.
Both Employees and Employers to See Increase in Healthcare Costs
The 2018 MMI report provides both good and bad news for the healthcare industry and patients. Milliman examined the costs for a typical family of four that participates in an employee-sponsored health insurance plan. For the report, a family of four consists of a 47-year old male, a 37-year old female, and two children under the age of five.
The MMI estimates a family of four will spend an average of $28,166 in healthcare expenditures in 2018. Included in this amount is the cost of the insurance paid by the employers and the employees, deductibles and out-of-pocket expenses. The figure represents an increase of $1,222 from 2017. The report found the amount families have been paying for healthcare has been increasing by an average of $100 per month over the last ten years.

The graphic above, taken from the 2018 Milliman Medical Index report, illustrates the increasing medical costs for a family of four. (Image copyright: Milliman.)
Both employers and employees will see an upsurge in costs from last year with employees experiencing an increase of 5.9% and employers seeing an increase of 3.5%.
The MMI found that employees will pay approximately 44% of their healthcare costs in 2018. By contrast, in 2008 employees paid less than 40% of their healthcare expenditures. In 2018, employers will pay about $15,788 of healthcare costs for a family of four, the employee will pay $7,674 via payroll deductions, with the remaining $4,704 being out-of-pocket expenses.
Costs Increasing While Growth Slows
The MMI also found that while the dollar amount families are spending on healthcare is increasing, the overall pace of the growth is slowing. The 4.5% rate of increase over last year is the slowest percentage growth in 18 years.
“We asked key stakeholders across the healthcare system what might be driving the decline in growth rates,” said Sue Hart, co-author of the MMI, in a Milliman news release. “Several common themes emerged, in particular provider engagement, more effective provider contracting, value-driven plan design, and spillover effects from public program initiatives.”
The reasons cited for this slowing trend include:
- Involvement of healthcare providers to reduce costs;
- More sophisticated contracting and provider consolidation;
- Increased member cost sharing;
- High deductible health plans;
- Role of government and public programs; and the,
- Impact of pharmacy initiatives.
“There are two ways of looking at this year’s MMI,” said Chris Girod, co-author of the Milliman Medical Index, in the news release. “On the one hand it’s heartening to see the rate of healthcare cost increase remain low. On the other hand, we’re still talking about more than $28,000 in total healthcare costs for the typical American family.”

The MMI graphic above breaks down healthcare costs into their constituent categories. (Image copyright: Milliman.)
To explore how costs have grown, the MMI examined five separate components of services. The typical family of four spends:
- 31% ($8,631) of their healthcare costs on inpatient facility care;
- 29% ($8,257) on professional services;
- 19% ($5,395) on outpatient facility care; and,
- 17% ($4,888) on pharmacy services.
The remaining 4% ($995) of costs are spent on other services, such as:
- Home healthcare;
- Ambulance services;
- Durable medical equipment; and,
- Prosthetics.
The MMI measures costs for a typical family of four, but certain families or individuals may have variations in costs depending on such factors as age, gender, health status, geographic area, provider variation, and insurance coverage.
Prescription drug costs is one such variance that is hard to predict. The 2018 MMI determined drug costs for a family of four increased by 6%, which represents the lowest percentage increase since 2015.
“Prescription drug costs have steadied, but this trend is volatile and hard to predict,” said Scott Weltz, co-author of the MMI in the news release. “High-cost drugs can have a big impact on trends, as we witnessed a few years ago when hepatitis C treatments hit the market. Alternatively, point-of-sale rebates could push a consumer’s costs in the other direction, particularly for people taking high-cost drugs. As the environment evolves, changes in drug prices can be deployed quite quickly.”

Scott Waltz (left), Christopher Girod (center), and Susan Hart (right) are Principles, Consulting Actuaries, for Milliman in Seattle. They co-authored the 2018 annual Milliman Medical Index report, which outlines the rising burden of out-of-pocket medical and insurance costs on patients, especially those on high deductible health plans. These costs are increasing and could impact clinical laboratories unprepared to collect fees at time of service. (Photo copyrights: Milliman.)
Preparing to Accept Payments
The results of this year’s MMI illustrate the impact increasing consumer costs could have on the way clinical laboratories conduct business and receive payments for services rendered. Studies have shown that patients with high deductible health plans (HDHPs), who frequently must pay 100% of lab costs, are especially affected by these trends. And the numbers of patients on HDHPs have increased each year since they were enacted.
Many clinical laboratories and anatomic pathology practices do not have the capability to collect fees from patients at the time of service. This lack of preparedness could threaten the survival of those labs and should be addressed.
—JP Schlingman
Related Information:
$28k: The Average Price a Family of Four Will Spend on Healthcare in 2018
2018 Milliman Medical Index
Milliman Medical Index: Healthcare Costs for Typical American Family Reach $28,166 Despite Low Annual Rate of Increase
Cost of Health Care for a Typical Family of Four Now over $28,000











