Oct 3, 2018 | Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
Next step is to design Web portal offering low-cost ‘polygenic risk score’ to people willing to upload genetic data received from DNA testing companies such as 23andMe
Pathologists and other medical professionals have long predicted that multi-gene diagnostics tests which examine thousands of specific gene sequences might one day hold the key to assessing disease risk, diagnosing diseases, and guiding precision medicine treatment decisions. Now, a research team from the Broad Institute, Massachusetts General Hospital (MGH) and Harvard Medical School have brought that prediction closer to reality.
Their study, published last month in Nature Genetics, found that a genome analysis called polygenic risk scoring can identify individuals with a high risk of developing one of five potentially deadly diseases:
- Coronary artery disease;
- Atrial fibrillation;
- Type 2 diabetes;
- Inflammatory bowel disease; and,
- Breast cancer.
Polygenic Scoring Predicts Risk of Disease Among General Population
To date, most genetic testing has been “single gene,” focusing on rare mutations in specific genes such as those causing sickle cell disease or cystic fibrosis. This latest research indicates that polygenic predictors could be used to discover heightened risk factors in a much larger portion of the general population, enabling early interventions to prevent disease before other warning signs appear. The ultimate goal of precision medicine.
“We’ve known for long time that there are people out there at high risk for disease based just on their overall genetic variation,” senior author Sekar Kathiresan, MD, co-Director of the Medical and Population Genetics Program at the Broad Institute, and Director, Center for Genomic Medicine at Massachusetts General Hospital, said in a Broad Institute news release. “Now, we’re able to measure that risk using genomic data in a meaningful way. From a public health perspective, we need to identify these higher-risk segments of the population, so we can provide appropriate care.”

“What I foresee is in five years, each person will know this risk number—this ‘polygenic risk score’—similar to the way each person knows his or her cholesterol,” Sekar Kathiresan, MD (above), Co-Director of the Medical and Population Genetics Program at the Broad Institute, and Director, Center for Genomic Medicine at Massachusetts General Hospital, told the Associated Press (AP). He went on to say a high-risk score could lead to people taking other steps to lower their overall risk for specific diseases, while a low-risk score “doesn’t give you a free pass” since an unhealthy lifestyle can lead to disease as well. (Photo copyright: Massachusetts General Hospital.)
The researchers conducted the study using data from more than 400,000 individuals in the United Kingdom Biobank. They created a risk score for coronary artery disease by looking for 6.6 million single-letter genetic changes that are more prevalent in people who have had early heart attacks. Of the individuals in the UK Biobank dataset, 8% were more than three times as likely to develop the disease compared to everyone else, based on their genetic variation.
In absolute terms, only 0.8% of individuals with the very lowest polygenic risk scores had coronary artery disease, compared to 11% for people with the highest scores, the Broad Institute news release stated.
“The results should be eye-opening for cardiologists,” Charles C. Hong, MD, PhD, Director of Cardiovascular Research at the University of Maryland School of Medicine, told the AP. “The only disappointment is that this score applies only to those with European ancestry, so I wonder if similar scores are in the works for the large majority of the world population that is not white.”
In its news release, the Broad Institute noted the need for additional studies to “optimize the algorithms for other ethnic groups.”
The Broad Institute’s results suggest, however, that as many as 25 million people in the United States may be at more than triple the normal risk for coronary artery disease. And millions more may be at similar elevated risk for the other conditions, based on genetic variations alone.
Reanalyzing Data from DNA Testing Companies
The researchers are building a website that would enable users to receive a low-cost polygenic risk score—such as calculating inherited risk score for many common diseases—by reanalyzing data users previously receive from DNA testing companies such as 23andMe.
Kathiresan told Forbes his goal is for the 17 million people who have used genotyping services to submit their data to the web portal he is building. He told the magazine he’s hoping “people will be able to get their polygenic scores for about as much as the cost of a cholesterol test.”
Some Experts Not Impressed with Broad Institute Study
But not all experts believe the Broad Institute/MGH/Harvard Medical School study deserves so much attention. Ali Torkamani, PhD, Director of Genomics and Genome Informatics at the Scripps Research Translational Institute, offered a tepid assessment of the Nature Genetics study.
In an article in GEN that noted polygenic risk scores were receiving “the type of attention reserved for groundbreaking science,” Torkamani said the recent news is “not particularly” a big leap forward in the field of polygenic risk prediction. He described the results as “not a methodological advance or even an unexpected result,” noting his own group had generated similar data for type 2 diabetes in their analysis of the UK dataset.
Nevertheless, Kathiresan is hopeful the study will advance disease treatment and prevention. “Ultimately, this is a new type of genetic risk factor,” he said in the news release. “We envision polygenic risk scores as a way to identify people at high or low risk for a disease, perhaps as early as birth, and then use that information to target interventions—either lifestyle modifications or treatments—to prevent disease.”
This latest research indicates healthcare providers could soon be incorporating polygenic risking scoring into routine clinical care. Not only would doing so mean another step forward in the advancement of precision medicine, but clinical laboratories and pathology groups also would have new tools to help diagnose disease and guide treatment decisions.
—Andrea Downing Peck
Related Information:
Genome-wide Polygenic Scores for Common Diseases Identify Individuals with Risk Equivalent to Monogenic Mutations
Predicting Risk for Common Deadly Diseases from Millions of Genetic Variants
Multigene Test May Find Risk for Heart Disease and More
A Harvard Scientist Thinks He Has a Gene Test for Heart Attack Risk. He Wants to Give It Away Free
Why Do Polygenic Risk Scores Get So Much Hype?
Oct 1, 2018 | Digital Pathology, Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
New advancements in mHealth, though encroaching on testing traditionally performed at clinical laboratories, offer opportunity to expand testing to remote locations
Mobile technology continues to impact clinical laboratories and anatomic pathology groups and is a major driver in precision medicine, as Dark Daily has reported. Most of the mobile-test development which incorporates smartphones as the testing device, however, has been for chemistry and immunoassay types of lab tests. Now, a new developer in Monmouth Junction, NJ, has created a Complete Blood Count (CBC) test that runs on devices attached to smartphones.
Such devices enable doctors to order test panels for patients in remote locations that also may lack resources, such as electricity.
The developer is Essenlix and it calls its new testing device iMOST (instant Mobile Self-Testing). According to the company’s website, which is mostly “Under Construction,” iMOST can provide “accurate blood and other healthcare testing in less than 60 seconds by a smartphone and matchbox-size-attachment, anywhere, anytime, and affordable to everyone.”
The company description on the Longitude Prize website states that Essenlix “uses multidisciplinary approaches to develop a new innovative platform of simple, fast, ultrasensitive, bio/chemical sensing and imaging for life science, diagnostics, and personal health.
The Longitude Prize competition was established to promote the invention of “an affordable, accurate, fast and easy-to-use test for bacterial infections that will allow health professionals worldwide to administer the right antibiotics at the right time,” the website states.

The Essenlix iMOST mobile-testing device (above) connects to a smartphone (shown right) and enables clinical laboratory technicians to run tests in remote locations from samples taken at time the test. Though still in trials, iMOST, and other similar devices, promise to expand testing to outside of traditional medical laboratory locations and further promote precision medicine. (Photos copyright: Lydia Ramsey/Business Insider.)
Essenlix’s iMOST mobile testing system consists of:
- a mobile application (app);
- the device attachment, which goes over the phone’s camera; and,
- a cartridge that holds a sample of blood.
So far, there have been two trials with a total of 92 participants, comparing traditional CBC testing with the Essenlix test. The results were within the FDA’s requirements for allowable error, prompting Chou to tell Business Insider, “Our error is clearly smaller than the FDA’s requirement, so the data is very, very good.”
Chou and his team are working toward FDA approval.
Other Testing Devices That Attached to Smartphones
Aydogan Ozcan, PhD, Professor of Electrical Engineering and Bioengineering at UCLA, and Mats Nilsson, PhD, Professor and Scientific Director of the Science for Life Laboratory at Stockholm University, have developed an attachment that they say can transform “a phone into a biomolecular analysis and diagnostics microscope,” according to The Pathologist. Dark Daily has published many e-briefings on Ozcan’s innovations over the years.
Their goal, the researchers said, was to create technology that can be used in low- and middle-income areas (LMICs), as well as in more advanced locations, such as Sweden. “I’ve been involved in other projects where we’ve looked at point-of-care diagnostic approaches,” he said, “and it seems to be very important that the devices [do not] rely on wired electricity or networks to serve not only LMICs, but also modern, developed environments. It’s often difficult to find an available power socket in Swedish hospitals.”
The molecular diagnostic tests that can be done with smartphone attachments—such as those developed by Ozcan and Nilsson—represent another way of using a smartphone in the healthcare arena, The Pathologist points out. Their invention combines the smartphone’s native camera, an app, optomechanical lasers, and an algorithm contained within the attachment to carry out fluorescence microscopy in the field.
Future of Mobile-Testing
An article appearing in the Financial Times describes some of the ways mobile technology is changing healthcare, including diagnostics that have traditionally been performed in the medical pathology laboratories.
“Doctors scan your body to look for irregularities, but they rely on pathologists in the lab to accurately diagnose any infection,” the article notes. “There, body fluids such as blood, urine, or spit are tested for lurking microbes or unexpected metabolites or chemicals wreaking havoc in your body. Now companies are miniaturizing these tests to create mobile pathology labs.”
Apple introduced the first iPhone in 2007. It’s doubtful anyone imagined the innovations in diagnostics and pathology that would soon follow. Thus, trying to predict what may be coming in coming decades—or even next year—would be futile. However, scientists and researchers themselves are indicating the direction development is headed.
Should Essenlix and other mobile-lab-test developers succeed in their efforts, it would represent yet another tectonic shift for medical pathology laboratories. Clinical laboratory managers and stakeholders should be ready, for the words of the ancient Greek philosopher Heraclitus have never been truer: “Change is the only constant in life.”
—Dava Stewart
Related Information:
Mobile Phone Microscopy
How Smartphones Are Transforming Healthcare
This Startup Wants to Make Blood Testing as Easy as Snapping a Photo with an iPhone
Is mHealth an Opportunity or Threat to Medical Laboratories and Pathology Groups?
New FDA Regulations of Clinical Decision-Support/Digital Health Applications and Medical Software Has Consequences for Medical Laboratories
UCLA Device Enables Diagnosis of Antimicrobial Resistance in Any Setting; Could Save Lives Lost to Antimicrobial Resistant Bacteria
Lab-on-a-Chip Diagnostics: When Will Clinical Laboratories See the Revolution?
Tiny, Simple-to-Use Lensless Microscope Might Soon Find a Place in Pathology
Sep 26, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
New metalens technology from MGH and SEAS researchers gives greater endoscopic optical imaging resolution and sample detail for anatomic pathologists performing diagnostics
Anatomic pathologists and clinical laboratories know that biopsy samples are necessary to diagnose many diseases. But, current endoscopic imaging techniques used by physicians sometimes fail to clearly visualize disease sites. Consequently, biopsies collected during these procedures may make it harder for pathologists and physicians to diagnose certain diseases and health conditions.
Now, a combined team of endoscopic imaging experts at Massachusetts General Hospital (MGH) and flat metalens developers at Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS) have developed “a new class of endoscopic imaging catheters—termed nano-optic endoscopes—that overcomes the limitations of current systems.”
That’s according to an article in Nature Photonics that reported on the research team’s study, published in Phys.org.
These nano-optics involved “flat metalenses” that promise to sharpen clarity and increase resolution of endoscopic imaging technology In turn, this contributes to more accurate pathology diagnostics and improve patient outcomes, while furthering the aims of precision medicine.
“Metalenses based on flat optics are a game changing new technology because the control of image distortions necessary for high resolution imaging is straightforward compared to conventional optics, which requires multiple complex shaped lenses,” Federico Capasso, PhD, Robert L. Wallace Professor of Applied Physics and Vinton Hayes Senior Research Fellow in Electrical Engineering at SEAS, and co-senior author of the study paper, told Nature Photonics. “I am confident that this will lead to a new class of optical systems and instruments with a broad range of applications in many areas of science and technology.”
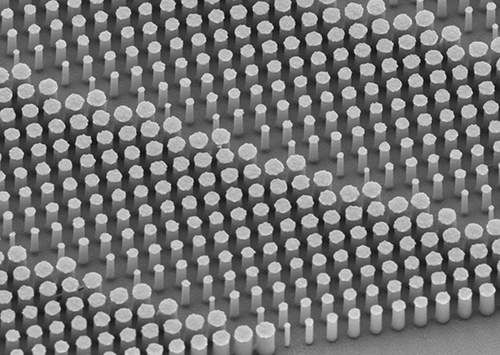
The image above shows a flat metalens taken using a scanning electron microscope. Anatomic pathologists and medical laboratories will benefit from the better quality biopsy specimens collected because of the sharper clarity and increased resolution of endoscopes built with the new nano-optics. (Photo copyright: Harvard SEAS.)
Researchers demonstrated the nano-optic endoscope’s ability to deeply penetrate and capture images at high resolutions in various tissues, including:
- Swine and sheep airways;
- Human lung tissue; and,
- Fruit flesh.
In the human lung tissue, “[T]he researchers were able to clearly identify structures that correspond to fine, irregular glands indicating the presence of adenocarcinoma, the most prominent type of lung cancer,” according to Phys.org.
Improving Endoscopic Imaging through Metalenses
The improved image resolution is due to the flat metalens configuration. “Currently, we are at the mercy of materials that we have no control over to design high-resolution lenses for imaging,” Yao-Wei Huang, PhD, Post-Doctoral Fellow at Harvard’s John A. Paulson School of Engineering and Applied Sciences and co-first author of the paper, told Phys.org.

Yao-Wei Huang, PhD (above), is a Post-Doctoral Fellow at Harvard’s John A. Paulson School of Engineering and Applied Sciences and co-first author of the study paper. “The main advantage of the metalens is that we can design and tailor its specifications to overcome spherical aberrations and astigmatism and achieve very fine focus of the light. As a result, we achieve very high resolution with extended depth of field without the need for complex optical components.” (Photo copyright: Harvard School of Engineering and Applied Sciences.)
The researchers note that current endoscopes using gradient-index (GRIN) lens-prism configurations and angle-polished ball lenses are used in a range of clinical applications due to their basic design. However, this benefit comes with shortfalls. “The ability of the nano-optic endoscope to obtain high-resolution images of sub-surface tissue structures in vivo is likely to increase the clinical utility of OCT [optical coherence tomography] in detection, diagnosis, and monitoring of diseases,” they state in their paper.
“The ability to control other properties of output light, such as the polarization state, enables a host of other applications—implausible to achieve using conventional catheters,” they continue. “Several tissues—such as smooth muscle, collagen (either innate or in fibrosis), and blood vessels—have constituent structures highly organized in one particular direction. Polarization-sensitive imaging can differentiate these structures from surrounding tissue by detecting their innate birefringence and optic axis.”
They further note that nano-optic endoscopes may yield benefits to other endoscopic optical imaging modalities such as confocal endomicroscopy.
Additional clinically-oriented studies will be required to assess how nano-optic endoscopes can elevate the capabilities of endoscopic OCT in examining fine pathological changes in luminal tissues.
Implications for Clinical Laboratories and Pathology Groups
The technology is still in the research stage with more trials needed to confirm the viability and accuracy of the approach. “This preclinical evaluation of the nano-optic endoscope indicated no significant flaws in the design for in vivo endoscopic imaging,” researchers note.
However, should nano-optic catheters gain clearance and change the endoscopy landscape as researchers predict, medical laboratories and pathologists might enjoy higher resolution images with greater information of the sample site—both key components of accurate diagnosis.
“Clinical adoption of many cutting-edge endoscopic microscopy modalities has been hampered due to the difficulty of designing miniature catheters that achieve the same image quality as bulky desktop microscopes,” Melissa Suter, PhD, Assistant Professor of Medicine at MGH and Harvard Medical School (HMS) and co-senior author of the study told Nature Photonics. “The use of nano-optic catheters that incorporate metalenses into their design will likely change the landscape of optical catheter design, resulting in a dramatic increase in the quality, resolution, and functionality of endoscopic microscopy. This will ultimately increase clinical utility by enabling more sophisticated assessment of cell and tissue microstructure in living patients.”
This research project at Massachusetts General Hospital and Harvard John A. Paulson School of Engineering and Applied Sciences is another example of how advances in technologies unrelated to surgical pathology can eventually contribute to improvements in how pathologists diagnose disease and help physicians identify the most promising therapies for their patients.
—Jon Stone
Related Information:
Nano-Optic Endoscope Sees Deep into Tissue at High Resolution
Nano-Optic Endoscope for High-Resolution Optical Coherence Tomography In Vivo
Nano-Optic Endoscope Allows High-Resolution Imaging
High-Resolution Nano-Optic Endoscope for Better Disease Detection
Sep 24, 2018 | Compliance, Legal, and Malpractice, Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations, News From Dark Daily
It’s the next wave in the long-running trend of hospital laboratory consolidation, as the need to trim costs and support thriving medical laboratory outreach programs continues
There’s an important new development in the hospital/health system sector of the clinical laboratory industry that continues the longstanding trend of consolidating multi-site lab operations. It is to rationalize and standardize medical laboratory operations across all lab sites within the health system. Effectively, this standardization trend represents the next cycle of clinical laboratory consolidation.
One recent example of this trend can be found at Atrium Health, the hospital health network based in Charlotte, N.C. (formerly known as Carolinas HealthCare System until earlier this year). Becker’s Hospital Review states that Atrium Health is the “seventh largest nonprofit system in the country based on number of acute-care hospitals (35).”
Creating Standardized Medical Laboratory Testing Services at Multiple Sites
Over the past four years, the clinical laboratory team at Atrium Health has worked to design, build, and operate a new, state-of-the-art core laboratory. At the same time, there were sequential projects to integrate the lab testing services and operations of nine other medical lab sites within the health system to better align the test menu, lab instruments, and workflow at these sites with the activities of the core laboratory.
According to Modena Henderson, MHA, the Vice President of Laboratory Services at Atrium Health, in an interview with Dark Daily, there were multiple primary goals in this project to rationalize and standardize lab testing at all the participating lab sites. They include:
- Standardizing lab test methodologies, reference ranges, and test menu;
- Standardizing analyzers and test platforms across all labs;
- Using Lean, Six Sigma, and other process improvement methods to streamline workflow and reduce test turnaround time;
- Improve productivity of lab staff;
- Increase quality while reducing or eliminating unproductive activities;
- Using real-time analytics middleware to keep lab management informed on a daily basis, and,
- Collaborating with emergency departments, wards, and outreach physicians to deliver more value with clinical lab testing services.
Using the ‘Three Ps of Project Management’ Approach in Health System Labs
The centerpiece of this program of lab rationalization and consolidation was the design and build-out for a new core clinical laboratory facility. Henderson said her team followed the principals of the “Three Ps of Project Management”—People, Process, Performance—to model the new lab facility, then guide how it was constructed and brought into daily clinical service.
“The Atrium Health laboratory regionalization project is an example of the next step that many innovative hospital laboratories are taking,” stated Robert L. Michel, Editor-in-Chief of The Dark Report. “Every lab has the same double challenge. First is financial. Hospital lab budgets are shrinking as growth in inpatient admissions slows. Outreach revenues are declining as Medicare and private payers slash lab test prices.
“Second, labs must come up with the capital needed to acquire and deploy the expensive and sophisticated new genetic and molecular tests that physicians and patients want,” he continued. “Hospital and health network labs must offer these new tests to keep their parent organizations at the cutting edge of clinical care.
Clinical Labs See Value in Standardizing Test Methodologies, Menus
“Thus, it is logical for the clinical labs of health networks to begin the process of rationalizing and standardizing their test menus, methodologies, and analyzers at every site within the system that performs medical lab testing,” emphasized Michel. “This is a development that we have watched gather momentum.”

Keynote Speaker Robert L. Michel, Editor-in-Chief of The Dark Report and Dark Daily will discuss how clinical laboratories of hospitals and health networks are rationalizing and standardizing their medical laboratory testing services to achieve the goals of managing lab costs, boosting quality, and increasing lab outreach revenue. The 12th annual Lab Quality Confab takes place on Oct. 9-10, 2018, at the Hyatt Regency Atlanta. (Photo copyright: The Dark Report.)
Michel offered two examples of sizable programs to rationalize and standardize clinical lab tests and services across a large health system. One is in Michigan, at Ascension Health. The other is in the Canadian Province of Québec. Both are large and ambitious undertakings, both in the number of lab sites involved and the large geography served by these clinical laboratories.
Consolidation Project in Québec involves 123 Clinical Lab Facilities
Québec’s provincial health system wants to consolidate 123 clinical laboratories in the province into 11 groups (clusters) of labs. Each lab group, or cluster, will have a core lab and rapid response labs. Test menus and methodologies will be standardized throughout the province. In an interview with The Dark Report, Ralph Dadoun, PhD, Project Director for Optilab Québec, plans to accomplish the consolidation without adding costs.
In Michigan, Ascension’s clinical lab leadership is working to integrate and standardize the labs that are operated by seven system organizations. This includes 14 hospitals and 18 existing laboratories located throughout the entire State of Michigan. In an interview with The Dark Report, Carlton Burgess, MSM, Vice President of Laboratory Services at Ascension Health’s St. John Providence Clinical Pathology Laboratory in Grosse Pointe Woods, Mich., stated that the goal is to have all the labs in the state work together in a seamless, integrated fashion.
Regional Lab Integration at North Carolina’s Biggest Health System
“To achieve this, the labs will be linked in four regions—a process we describe as regional integration,” explained Burgess. “Each region has a core lab and rapid response labs and each region will be responsible for building lab volume through increased outreach testing. In addition to changing how labs serve each region, our statewide standardization project has three objectives:
- “Repatriate existing send-out lab testing back into Michigan;
- “Establish standard test menus for each facility; and,
- “Renew each lab’s focus on growing lab outreach business.
“Every lab administrator and pathologist working in hospital and health network laboratories should be tracking this new trend of regionalization and standardization of hospital labs,” observed Michel. “That’s because labs already moving down this path are setting new standards for the entire clinical laboratory industry. This goes beyond cost and productivity, because these labs are putting the systems in place that will allow them to deliver more value to physicians and thus be paid more for that value by private health insurers.”
Innovative Lab Leaders to Speak at Lab Quality Confab in Atlanta
Lab leaders from Ascension Health will be keynote speakers at the upcoming 12th Annual Lab Quality Confab that takes place on October 9-10, 2018, at the Hyatt Hotel in Atlanta. They will also conduct multiple learning sessions to share their successes and lessons learned in building a new core laboratory and using that as a foundation to rationalize and standardize test methods, reference ranges, menus, lab automation, and analyzers at every clinical lab facility in the Ascension Health system. Sessions by Ascension Health lab leaders include:
- Leveraging Lean to become a Best-in-Class Lab Performer: How We Built and Automated a New Core Lab while Integrating Lab Operations and Helping Staff Embrace a New Culture; Modena Henderson, Vice President, Laboratory Services, and, Steven Harris, Assistant Vice President, Atrium Health.
- Achieving Standardized, High-Performance Lab Testing Services at Multiple Hospitals Using Lean Methods and Effective Engagement with Lab Staff and Nurses; Gary Catarella, MBA, MT(ASCP), Assistant Vice President, Hospital Operations, Atrium Health.
- Lessons We’ve Learned in Our Step-by-Step Journey to Transform Lab Operations and Integrate Testing across All Sites: Engaging Staff, Sustaining Change, Working with Vendors and Consultants—Interactive Roundtable Discussion; Modena Henderson, Vice President, Laboratory Services; and, Steven Harris, Assistant Vice President, Atrium Health.
Using Lean, Six, Sigma, ISO 15189 in Clinical Laboratory Operations
Lab Quality Confab this year features 60 speakers and 40 presentations from lab administrators, pathologists, and other lab managers on their successes and innovations using Lean, Six Sigma, ISO 15189, and other process management methods. You can view the full agenda here (or copy and paste this URL into your web browser: https://www.labqualityconfab.com/agenda).
This year’s Lab Quality Confab is on track to be the largest in its 12-year history. Limited spaces are still available. To ensure your place, register today at: https://www.labqualityconfab.com/register (or copy and paste this URL into your web browser: https://www.labqualityconfab.com/register).
Also, you can bring your lab team and make this Lab Quality Confab a group learning opportunity. When you bring four or more from your organization, each can register for $695 for this two-day learning event. One benefit you’ll gain from bringing your team is that it will give them the knowledge, the tools, and the confidence to help your lab reduce costs without compromising quality, while supporting sustained revenue growth from your hospital lab’s successful outreach program.
—Michael McBride
Related Information:
Full Agenda and Other Details for 12th Annual Lab Quality Confab
To Register for 12th Annual Lab Quality Confab
10 Things to Know about Atrium Health, Formerly Carolinas HealthCare System
Québec’s Laboratory Consolidation Plan Aims to Save $13.5 Million: Optilab Québec to move 123 labs into 11 lab groups
Michigan’s Ascension to Standardize Labs Throughout the State: Goals Are Common Test Methods, Menus, Practices
Sep 21, 2018 | Instruments & Equipment, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing
Wall Street Journal reports IBM losing Watson-for-Oncology partners and clients, but scientists remain confident artificial intelligence will revolutionize diagnosis and treatment of disease
What happens when a healthcare revolution is overhyped? Results fall short of expectations. That’s the diagnosis from the Wall Street Journal (WSJ) and other media outlets five years after IBM marketed its Watson supercomputer as having the potential to “revolutionize” cancer diagnosis and treatment.
The idea that artificial intelligence (AI) could be used to diagnose cancer and identify appropriate therapies certainly carried with it implications for clinical laboratories and anatomic pathologists, which Dark Daily reported as far back as 2012. It also promised to spark rapid growth in precision medicine. For now, though, that momentum may be stalled.
“Watson can read all of the healthcare texts in the world in seconds,” John E. Kelly III, PhD, IBM Senior Vice President, Cognitive Solutions and IBM Research, told Wired in 2011. “And that’s our first priority, creating a ‘Dr. Watson,’ if you will.”
However, despite the marketing pitch, the WSJ investigation published in August claims IBM has fallen far short of that goal during the past seven years. The article states, “More than a dozen IBM partners and clients have halted or shrunk Watson’s oncology-related projects. Watson cancer applications have had limited impact on patients, according to dozens of interviews with medical centers, companies and doctors who have used it, as well as documents reviewed by the Wall Street Journal.”
Anatomic pathologists—who use tumor biopsies to diagnose cancer—have regularly wondered if IBM’s Watson would actually help physicians do a better job in the diagnosis, treatment, and monitoring of cancer patients. The findings of the Wall Street Journal show that Watson has yet to make much of a positive impact when used in support of cancer care.
The WSJ claims Watson often “didn’t add much value” or “wasn’t accurate.” This lackluster assessment is blamed on Watson’s inability to keep pace with fast-evolving treatment guidelines, as well as its inability to accurately evaluate reoccurring or rare cancers. Despite the more than $15 billion IBM has spent on Watson, the WSJ reports there is no published research showing Watson improving patient outcomes.
Lukas Wartman, MD, Assistant Professor, McDonnell Genome Institute at the Washington University School of Medicine in St. Louis, told the WSJ he rarely uses the Watson system, despite having complimentary access. IBM typically charges $200 to $1,000 per patient, plus consulting fees in some cases, for Watson-for-Oncology, the WSJ reported.
“The discomfort that I have—and that others have had with using it—has been the sense that you never know how much faith you can put in those results,” Wartman said.
Rudimentary Not Revolutionary Intelligence, STAT Notes
IBM’s Watson made headlines in 2011 when it won a head-to-head competition against two champions on the game show “Jeopardy.” Soon after, IBM announced it would make Watson available for medical applications, giving rise to the idea of “Dr. Watson.”
In a 2017 investigation, however, published on STAT, Watson is described as in its “toddler stage,” falling far short of IBM’s depiction of Watson as a “digital prodigy.”
“Perhaps the most stunning overreach is in [IBM’s] claim that Watson-for-Oncology, through artificial intelligence, can sift through reams of data to generate new insights and identify, as an IBM sales rep put it, ‘even new approaches’ to cancer care,” the STAT article notes. “STAT found that the system doesn’t create new knowledge and is artificially intelligent only in the most rudimentary sense of the term.”
STAT reported it had taken six years for data engineers and doctors to train Watson in just seven types of cancers and keep the system updated with the latest knowledge.
Watson Recommended Unsafe and Incorrect Treatments, STAT Reported
In July 2018, STAT reported that internal documents from IBM revealed Watson had recommended “unsafe and incorrect” cancer treatments.
David Howard, PhD, Professor, Health Policy and Management, Rollins School of Public Health at Emory University, blames Watson’s failure in part to the dearth of high-quality published research available for the supercomputer to analyze.
“IBM spun a story about how Watson could improve cancer treatment that was superficially plausible—there are thousands of research papers published every year and no doctor can read them all,” Howard told HealthNewsReview.org. “However, the problem is not that there is too much information, but rather there is too little. Only a handful of published articles are high-quality, randomized trials. In many cases, oncologists have to choose between drugs that have never been directly compared in a randomized trial.”
Howard argues the news media needs to do a better job vetting stories touting healthcare breakthroughs.
“Reporters are often susceptible to PR hype about the potential of new technology—from Watson to ‘wearables’—to improve outcomes,” Howard said. “A lot of stories would turn out differently if they asked a simple question: ‘Where is the evidence?’”
Peter Greulich, a retired IBM manager who has written extensively on IBM’s corporate challenges, told STAT that IBM would need to invest more money and people in the Watson project to make it successful—an unlikely possibility in a time of shrinking revenues at the corporate giant.
“IBM ought to quit trying to cure cancer,” he said. “They turned the marketing engine loose without controlling how to build and construct a product.”
AI Could Still Revolutionize Precision Medicine
Despite the recent negative headlines about Watson, AI continues to offer the promise of one day changing how pathologists and physicians work together to diagnose and treat disease. Isaac Kohane, MD, PhD, Chairman of the Biomedical Informatics Program at Harvard Medical School, told Bloomberg that IBM may have oversold Watson, but he predicts AI one day will “revolutionize medicine.”
“It’s anybody’s guess who is going to be the first to the market leader in this space,” he said. “Artificial intelligence and big data are coming to doctors’ offices and hospitals. But it won’t necessarily look like the ads on TV.”
How AI and precision medicine plays out for clinical laboratories and anatomic pathologists is uncertain. Clearly, though, healthcare is on a path toward increased involvement of computerized decision-making applications in the diagnostic process. Regardless of early setbacks, that trend is unlikely to slow. Laboratory managers and pathology stakeholders would be wise to keep apprised of these developments.
—Andrea Downing Peck
Related Information:
IBM’s Watson Supercomputer Recommended ‘Unsafe and Incorrect’ Cancer Treatments, Internal Documents Show
IBM Pitched its Watson Supercomputer as a Revolution in Cancer Care. It’s Nowhere Close
IBM’s Watson Wins Jeopardy! Next Up: Fixing Health Care
IBM’s Watson Supercomputer Wins Practice Jeopardy Round
Memorial Sloan-Kettering Cancer Center, IBM to Collaborate in Applying Watson Technology to Help Oncologists
IBM Has a Watson Dilemma
MD Anderson Cancer Center’s IBM Watson Project Fails, and So Did the Journalism Related to It
What Went Wrong with IBM’s Watson?
IBM’s Watson Failed Against Cancer but AI Still Has Promise
Will IBM’s ‘Watson on Oncology’ Give Oncologists and Pathologists a Useful Tool for Diagnosing and Treating Various Cancer
Pathologists Take Note: IBM’s Watson to Attack Cancer with Help of WellPoint and Cedars-Sinai
Sep 19, 2018 | Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
As inpatient care declines, outpatient care expands, signaling a shift from where physician orders for medical laboratory tests originate
It’s a well-established fact that the year-over-year increase in the volume of outpatient procedures is consistently greater than 8%, while the annual growth in inpatient admissions is at or below 3%. Clinical laboratory managers and anatomic pathologists are aware of these facts.
MEDACorp, a strategic knowledge resource and subsidiary of Leerink Partners, a Boston-based investment bank that focuses on the healthcare industry, conducted a quarterly survey of hospital administrators from 47 hospitals in June of this year. The purpose was to evaluate healthcare utilization trends for different types of medical facilities.
According to the survey, the “ utilization trends for both [quarter-over-quarter] and [year-over-year] are decelerating across all four sites of service.” However, hospital administrators report the use of inpatient and other hospital services did not fare as well.
“The results are also modestly positive for facilities, with the slowing 0.7% increase in inpatient procedures (IP) utilization still higher than consensus of ~flat (sic) for the coverage group. Significant mix shift continues as IP mix is down and the outpatient procedure (OP) mix is up across all service lines, supporting our recent analyses of an accelerated push to ambulatory sites of service,” the survey noted.
The survey revealed:
- Inpatient services increased by just 0.7% during the second quarter of 2018, down from 1% growth during the second quarter of 2017;
- Ambulatory surgery center utilization grew by just 1.4% during the second quarter of 2018, down from 2.2% for the same quarter in 2017; and,
- Emergency department utilization increased by 0.9% in the second quarter of 2018.

“It was even weaker than many of us expected,” Ana Gupte, PhD, Managing Director, Healthcare Services at Leerink, told Modern Healthcare. “It’s pretty clear there’s no volume rebound.” (Photo copyright: Leerink.)
Inpatient Top Surgeries Predicted to Keep Moving to Outpatient Settings
The Leerink survey found that orthopedic and cardiac procedures are leading the trend of shifting clinical procedures from inpatient to outpatient care. For example, heart surgeries were performed in outpatient settings 9% of the time over the past year and are expected to increase to 12% during the next year. Inpatient heart surgeries are expected to decrease 1% over the next year, moving from 82% to 81% of surgeries performed.
Inpatient hip procedures also are projected to drop from 77% to 75% over the course of the next year, while outpatient hip procedures are expected to increase from 17% to 19%. The survey respondents also expect other surgeries including urological, gynecological, spine and knee procedures to move to outpatient settings over the next year.
Forty-five percent of the respondents in the survey named baby boomers aging into Medicare as the most important driver of hospital utilization. Fifteen percent attributed the trend to the effects of the Affordable Care Act (ACA), and 13% cited the improving US economy as driving the use of outpatient medical facilities.
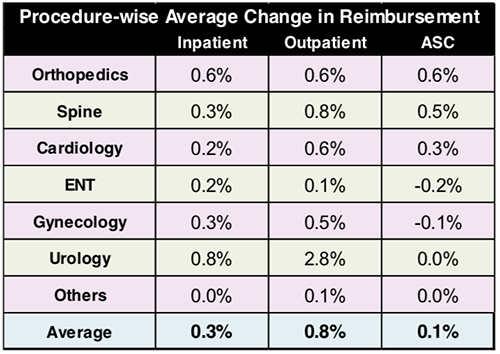
The graphic above, taken from the Leerink MEDACorp survey, illustrates a shift in healthcare procedures from inpatient to outpatient settings. Along with the surgical relocations are changes in reimbursements as well, which directly impacts clinical laboratories revenue streams and billing procedures. (Image copyright: Leerink.)
The survey respondents were from different regions and disparate population areas:
- 49% of the surveyed facilities were located in metropolitan areas;
- 40% were in suburban areas; and,
- 11% were in rural areas.
In addition:
- 32% were in the South;
- 26% were in the Northeast;
- 21% were in the Midwest; and,
- 21% were in the West.
Most of the facilities used in the survey were community hospitals, which accounted for 62% of the environments. Thirty-two percent of the facilities were academic medical centers, while the remaining 6% were other types of facilities.
The majority of the respondents for the survey were C-suite executives. In hospital settings that would include the Chief Executive Officer (CEO), Chief Financial Officer (CFO), Chief Operating Officer (COO), and other Chief titles, and, of course, hospital administrators.
Clearly, this type of downward trend could negatively impact clinical laboratories, pathology groups, and other service lines in hospital networks. Fewer patients equal less revenue for hospitals, which could eventually lead to budget cuts. A responsible medical laboratory manager will be prepared for these possibilities.
—JP Schlingman
Related Information:
Hospital Execs Say Inpatient Volume Growth Isn’t Rebounding
Hospital Utilization Slows as Healthcare Moves to Cheaper Care Settings
2Q18 Hospital Util. Survey: Utilization Decelerating, Shift To OP/ ASC Continues
ASCs Beat Inpatient Setting for Quarter, Year-over-year Utilization Increases—6 Study Insights
10 Key Trends for ASCs and Outpatient Surgery in the Next 10 Years