Nov 9, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Pathology
Studies show consumer genealogy databases are much broader than is generally known. If your cousins are in such a database, it’s likely you are too
Recent news stories highlighted crime investigators who used the DNA data in consumer genetic genealogy databases to solve cold cases. Though not widely known, such uses of direct-to-consumer DNA databases is becoming more commonplace, which might eventually lead to requests for clinical laboratories to assist in criminal investigations involving DNA data.
Case in point: investigators found the Golden State Killer, a serial killer/rapist/burglar who terrorized multiple California counties over a dozen years in the 1970s to 1980s, after uploading a DNA sample from the crime scene to GEDmatch, an open-data genomics database that features tools for genealogy research. They made the arrest after discovering a distant relative’s DNA in the genealogy database and matching it to the suspect, CBS News revealed in a 60 Minutes Overtime online report.
These and other investigators are using a technique called familial DNA testing (AKA, DNA Profiling), which enables them to use genetic material from relatives to solve crimes.
Clinical laboratories oversee DNA databases. Could DNA databases—developed and managed over years by medical laboratories for patient care—be subpoenaed by law enforcement investigating crimes?
The question raises many issues for society and for labs, including privacy responsibilities and appropriate use of genetic information. On the other hand, the genetic genie is already out of the bottle.
Leveraging Familia DNA to Solve Crimes a New Trend
“The solving of the Golden State Killer case opened this method up as a possibility, and other crime labs are taking advantage of it. Clearly, a trend has started,” Ruth Dickover, PhD, Director of Forensic Science, University of California, Davis, told the Los Angeles Times.
Indeed, the use of familial DNA testing is moving forward. The Verge reported 19 cold case samples have been identified in recent familial DNA testing and public database searches. It also said two new published studies may propel the technique further.
One study, published in the journal Science, suggests nearly every American of European ancestry may soon be identified through familial DNA testing.
The other study, published in Cell, shows that a person’s relatives can be detected when forensic DNA data are compared with consumer genetic databases.

Noah Rosenberg, PhD (above left), Professor of Population Genetics and Society Biology at Stanford University, is shown above working with Jaehee Kim, PhD (right), a Postdoctoral Research Fellow in Biology, on math that could be used to track down relatives in genealogy databases based on forensic DNA. “This could be a way of expanding the reach of forensic genetics, potentially for solving even more cold cases. But at the same time, it could be exposing participants in those databases to forensic searches they might not have anticipated,” he told Wired. (Photo copyright: Stanford University/L.A. Cicero.)
15 Million People Already in Genealogy Databases
Researchers at Columbia University in New York and Hebrew University of Jerusalem told Science they were motivated by the recent trend of investigations leveraging third-party consumer genomics services to find criminals. But they perceived a gap.
“The big limitation is coverage. And even if you find an individual it requires complex analysis from that point,” Yaniv Erlich, PhD, Associate Professor at Columbia and Chief Science Officer at MyHeritage, told The Verge. MyHeritage is an online genealogy platform.
Others offering consumer genetic testing and family history exploration include 23andMe and Ancestry. As of April 2018, more than 15 million people have participated in direct-to-consumer genetic testing, the researchers noted.
The study aimed to find the likelihood that a person can be identified using a long-range familial search. It included these steps and findings:
- Statistical analysis of 1.28 million people in the MyHeritage database;
- Pairs of people with “identity-by-descent” were removed to avoid bias, such as first cousins and closer relationships;
- Researchers aimed at finding a third cousin or closer relatives for each person in the database;
- 60% of the 1.28 million people were matched with a third cousin or closer relative.
“We project that about 60% of the searches for individuals of European-descent will result in a third cousin or closer match, which can allow their identification using demographic identifiers. Moreover, the technique could implicate nearly any US individual of European descent in the near future,” the researchers wrote.
In an interview with Wired, Erlich added, “The takeaway is it doesn’t matter if you’ve been tested or not tested. You can be identified because the databases already cover such large fractions of the US—at least for European ancestry.”
Matching Forensic and Consumer Genetic Data
Meanwhile, the study published in Cell by researchers at Stanford University, University of California, Davis, and the University of Michigan also suggests investigators could compare forensic DNA samples with consumer genetic databases to find people related to criminals.
That study found:
- 30% to 32% of people in a forensic database could be related to a child or parent in a consumer database;
- 35% to 36% could be tied to a sibling.
These studies reveal that genetic data and familial DNA testing can help law enforcement find suspects, which is a good thing for society. But people who uploaded DNA data to some direct-to-consumer databases may find themselves caught up in searches they do not know about. So may their cousins.
Dark Daily recently covered other similar studies that showed it takes just one person’s DNA to reveal genetic information on an entire family. (See, “The Problems with Ancestry DNA Analyses,” October 18, 2018.) These developments in the use of DNA databases to identify criminals should be an early warning to clinical laboratories building databases of genetic information that, at some future point, law enforcement agencies might want access to those databases as part of ongoing criminal investigations.
—Donna Marie Pocius
Related Information:
Could Your DNA Help Solve a Cold Case?
So Many People Have Had Their DNA Sequenced That They’ve Put Other People’s Privacy in Jeopardy
The DNA Technique That Caught the Golden State Killer is More Powerful than We Thought
Identity Inference of Genomic Data Using Long-Range Familial Searches
Statistical Detection of Relatives Typed with Disjoint Forensic and Biomedical Loci
Genome Hackers Show No One’s DNA is Anonymous Anymore
Stanford Researchers Discover a New Way to Find Relatives from Forensic DNA
The Problems with Ancestry DNA Analyses
Nov 5, 2018 | Digital Pathology, Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing
Computer-assisted analysis using Google’s LYNA algorithm shows significant gains in speed required to analyze stained lymph node slides and sensitivity of micrometastases detection in two recent studies
Anatomic pathologists understand the complexities of reviewing slides and samples for signs of cancer’s spread. Two studies involving a new artificial intelligence (AI) algorithm from Google (NASDAQ:GOOGL) claim their “deep learning” LYmph Node Assistant (LYNA) provides increases to both the speed at which pathologists can analyze slides and improved accuracy in detecting metastatic breast cancer within the slide samples used for the studies.
Google’s first study was published in the Archives of Pathology and Laboratory Medicine and investigated the accuracy of the algorithm using digital pathology slides. Google’s second study, published in The American Journal of Surgical Pathology, looked at how pathologists might harness the algorithm to improve workflows and use the tool in a clinical setting.
Medical laboratories and other diagnostics providers are already familiar with the improvement potential of automation and other technology-based approaches to diagnosis and analysis. Google’s LYNA is an example of how AI and machine learning improvements can serve as a supplement to—not a replacement for—the skills of experts at pathology groups and clinical laboratories.
Early research done by Google indicates that integrating LYNA into existing workflows could allow pathologists to spend less time analyzing slides for minute details. Instead, they could focus on other more challenging tasks while the AI analyzes gigapixels worth of slide data to highlight regions of concern in slides and samples for deeper manual inspection.
LYNA Achieves 99% Accuracy in Study of Metastatic Breast Cancer Detection
According to the research cited in a Google AI Blog post, roughly 25% of metastatic lymph node staging classifications would change if subjected to a second pathologic review. They further note that when faced with time constraints, detection sensitivity for small metastases on individual slides can be as low as 38%.
In findings published in Archives of Pathology and Laboratory Medicine, Google researchers analyzed whole slide images from hematoxylin-eosin-stained lymph nodes for 399 patients sourced from the Camelyon16 challenge dataset. Of those slides, researchers used 270 to train LYNA and the remaining 129 for analysis. They then compared the LYNA findings to those of an independent lab using a different scanner.
“LYNA achieved a slide-level area under the receiver operating characteristic (AUC) of 99% and a tumor-level sensitivity of 91% at one false positive per patient on the Camelyon16 evaluation dataset,” the researchers stated. “We also identified [two] ‘normal’ slides that contained micrometastases.”
Google’s algorithm later received an AUC of 99.6% on a secondary dataset.
“Artificial intelligence algorithms can exhaustively evaluate every tissue patch on a slide, achieving higher tumor-level sensitivity than, and comparable slide-level performance to, pathologists,” the researchers continued. “These techniques may improve the pathologist’s productivity and reduce the number of false negatives associated with morphologic detection of tumor cells.”
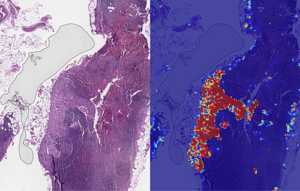
Left: sample view of a slide containing lymph nodes, with multiple artifacts: the dark zone on the left is an air bubble, the white streaks are cutting artifacts, the red hue across some regions are hemorrhagic (containing blood), the tissue is necrotic (decaying), and the processing quality was poor. Right: LYNA identifies the tumor region in the center (red), and correctly classifies the surrounding artifact-laden regions as non-tumor (blue). (Image and caption copyright: Google AI Blog.)
Faster Analysis through Software Assistance
Rapid diagnosis helps improve cancer outcomes. Yet, manually reviewing and analyzing complex digital slides is time-consuming. Time constraints might also lead to false negatives due to micrometastases or small suspicious regions that slip by pathologists undetected.
The Google research team of the study published in The American Journal of Surgical Pathology sought to gauge the impact LYNA might have on the histopathologic review of lymph nodes for trained pathologists. In their multi-reader multi-case study, researchers analyzed differences in both sensitivity of detecting micrometastases and the average review time per image using both computer-aided detection and unassisted detection for six pathologists across 70 slides.
Using the LYNA algorithm to identify and outline regions likely to contain tumors, the researchers found that sensitivity increased from 83% to 91%. The time to review slides also saw a significant reduction from 116 seconds in the unassisted mode to 61 seconds in the assisted mode—a time savings of roughly 47%.
“Although some pathologists in the unassisted mode were less sensitive than LYNA,” the researchers stated, “all pathologists performed better than the algorithm alone in regard to both sensitivity and specificity when reviewing images with assistance.”
The Future of Digital Pathology using LYNA
While the two studies show positive results, both studies also reveal shortcomings. Google highlighted both limited dataset sizes and simulated diagnostic workflows as potential concerns and areas on which to focus future studies.
Still, Google’s researchers believe that algorithms such as LYNA will help to power the future of diagnostics as healthcare in the digital era continues to mature. “We remain optimistic,” state the authors of the Google AI Blog post, “that carefully validated deep learning technologies and well-designed clinical tools can help improve both the accuracy and availability of pathologic diagnosis around the world.”
While other industries see risk in the growth of AI, both studies performed by researchers at Google show how computer-assisted workflows and machine learning could accentuate and bolster the skills of trained diagnosticians, such as anatomic pathologists and clinical laboratory technicians. By working to compensate for weak points in both human skill and computer reasoning, the outcome could be greater than either AI or humans can achieve separately.
—Jon Stone
Related Information:
Google Creates AI to Detect When Breast Cancer Spreads
Google Deep Learning Tool 99% Accurate at Breast Cancer Detection
Google’s AI Software Seeks to Detect Advanced Breast Cancer Better Than We Have Before
Google’s AI Is Better at Spotting Advanced Breast Cancer than Pathologists
Google AI Claims 99% Accuracy in Metastatic Breast Cancer Detection
Applying Deep Learning to Metastatic Breast Cancer Detection
Assisting Pathologists in Detecting Cancer with Deep Learning
Diagnostic Assessment of Deep Learning Algorithms for Detection of Lymph Node Metastases in Women with Breast Cancer
Nov 2, 2018 | Coding, Billing, and Collections, Compliance, Legal, and Malpractice, Digital Pathology, Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
Patient privacy, ethics of monetizing not-for-profit data, and questions surrounding industry conflicts appear after the public announcement of an arrangement to grant exclusive access to academic pathology slides and samples
Clinical laboratories and anatomic pathology groups already serve as gatekeepers for a range of medical data used in patient treatments. Glass slides, paraffin-embedded tissue specimens, pathology reports, and autopsy records hold immense value to researchers. The challenge has been how pathologists (and others) in a not-for-profit academic center could set themselves up to potentially profit from their exclusive access to this archived pathology material.
Now, a recent partnership between Memorial Sloan Kettering Cancer Center (MSK) and Paige.AI (a developer of artificial intelligence for pathology) shows how academic pathology laboratories might accomplish this goal and serve a similar gatekeeper role in research and development using the decades of cases in their archives.
The arrangement, however, is not without controversy.
New York Times, ProPublica Report
Following an investigative report from the New York Times (NYT) and ProPublica, pathologists and board members at MSK are under fire from doctors and scientists there who have concerns surrounding ethics, exclusivity, and profiting from data generated by physicians and but owned by MSK.
“Hospital pathologists have strongly objected to the Paige.AI deal, saying it is unfair that the founders received equity stakes in a company that relies on the pathologists’ expertise and work amassed over 60 years. They also questioned the use of patients’ data—even if it is anonymous—without their knowledge in a profit-driven venture,” the NYT article states.
Prominent members of MSK are facing scrutiny from the media and peers—with some relinquishing stakes in Paige.AI—as part of the backlash of the report. This is an example of the perils and PR concerns lab stakeholders might face concerning the safety of data sharing and profits made by medical laboratories and other diagnostics providers using patient data.
Controversy Surrounds Formation of Paige.AI/MSK Partnership
In February 2018, Paige.AI announced closing the deal on a $25-million round of Series A funding, and in gaining exclusive access to 25-million pathology slides and computational pathology intellectual property held by the Department of Pathology at Memorial Sloan Kettering. Coverage by TechCrunch noted that while MSK received an equity stake as part of the licensing agreement, they were not a cash investor.
Creation of the company involved three hospital insiders and three additional board members with the hospital itself established as part owner, according to STAT.
Unnamed officials told the NYT that board members at MSK only invested in Paige.AI after earlier efforts to generate outside interest and investors were unsuccessful. NYT’s coverage also notes experts in non-profit law and corporate governance have raised questions as to compliance with federal and state laws that govern nonprofits in light of the Paige.AI deal.
Growing Privacy Fallout and Potential Pitfalls for Medical Labs
The original September 2018 NYT coverage noted that Klimstra intends to divest his ownership stake in Paige.AI. Later coverage by NYT in October, notes that Democrat Representative Debbie Dingell of Michigan submitted a letter questioning details about patient privacy related to Paige.AI’s access to MSK’s academic pathology resources.
Privacy continues to be a focus for both media and regulatory scrutiny as patient data continues to fill electronic health record (EHR) systems as well as research and commercial databases. Dark Daily recently covered how University of Melbourne researchers demonstrated how easily malicious parties might reidentify deidentified data. (See “Researchers Easily Reidentify Deidentified Patient Records with 95% Accuracy; Privacy Protection of Patient Test Records a Concern for Clinical Laboratories”, October 10, 2018.)
According to the NYT, MSK also issued a memo to employees announcing new restrictions on interactions with for-profit companies with a moratorium on board members investing in or holding board positions in startups created within MSK. The nonprofit further noted it is considering barring hospital executives from receiving compensation for their work on outside boards.
However, MSK told the NYT this only applies to new deals and will not affect the exclusive deal between Paige.AI and MSK.
“We have determined,” MSK wrote, “that when profits emerge through the monetization of our research, financial payments to MSK-designated board members should be used for the benefit of the institution.”
There are no current official legal filings regarding actions against the partnership. Despite this, the arrangement—and the subsequent fallout after the public announcement of the arrangement—serve as an example of pitfalls medical laboratories and other medical service centers considering similar arrangements might face in terms of public relations and employee scrutiny.
Risk versus Reward of Monetizing Pathology Data
While the Paige.AI situation is only one of multiple concerns now facing healthcare teams and board members at MSK, the events are an example of risks pathologists take when playing a role in a commercial enterprise outside their own operations or departments.
In doing so, the pathologists investing in and shaping the deal with Paige.AI brought criticism from reputable sources and negative exposure in major media outlets for their enterprise, themselves, and MSK as a whole. The lesson from this episode is that pathologists should tread carefully when entertaining offers to access the patient materials and data archived by their respective anatomic pathology and clinical laboratory organizations.
—Jon Stone
Related Information:
Sloan Kettering’s Cozy Deal with Start-Up Ignites a New Uproar
Paige.AI Nabs $25M, Inks IP Deal with Sloan Kettering to Bring Machine Learning to Cancer Pathology
Sloan Kettering Executive Turns Over Windfall Stake in Biotech Start-Up
Cancer Center’s Board Chairman Faults Top Doctor over ‘Crossed Lines’
Memorial Sloan Kettering, You’ve Betrayed My Trust
LVHN Patient Data Not Shared with For-Profit Company in Sloan Kettering Trials
Researchers Easily Reidentify Deidentified Patient Records with 95% Accuracy; Privacy Protection of Patient Test Records a Concern for Clinical Laboratories
Oct 31, 2018 | Laboratory Hiring & Human Resources, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing
Only 3% of histopathology departments that responded to the Royal College of Pathologists’ workforce census reported enough staff to meet clinical demand
There is a chronic shortage of histopathologists in the United Kingdom (UK) and it is being blamed for cancer treatment waiting times that now reach the worst-ever levels, as National Health Service (NHS) training initiatives and other steps fail to keep pace with growing demand for diagnostic services.
For US anatomic pathologists and clinical laboratory managers, headlines from the UK reveal the impact a shortage of trained histopathologists (UK’s version of anatomic pathologists) and support technical staff can have on patient care when longer wait times for pathology support and diagnosis become the norm.
Royal College of Pathologists Report
The extent of the UK-wide histopathology staff shortages was highlighted in a recently released 2017 workforce census by the Royal College of Pathologists (RCPath). Of the 103 histopathology departments that responded to a survey, only 3% said they had enough staff to meet the current clinical demand! And 45% of departments had to outsource work, while half of the departments were forced to use more expensive temporary workers.
“The cost of staff shortages across histopathology departments is high for both patients and for our health services,” Jo Martin, PhD, President of the Royal College of Pathologists, noted in a statement announcing the survey results. “For patients, it means worrying delays in diagnosis and treatment. For NHS hospitals, it means spending more resources on [temporary] doctors to fill staffing gaps, or outsourcing services. We estimate this cost [to be] £27 million ($35.2 million) each year across the UK health service—money that could be better invested in staff and new diagnostic equipment.”

Royal College of Pathologists President Jo Martin, PhD, is calling on the National Health Service to take additional steps to increase the number of pathologists working in the United Kingdom, including more funded training places. That’s following the release of a Royal College of Pathologists workforce survey, which reported only 3% of histopathology departments in the UK have enough staff to meet clinical demand. (Photo copyright: Twitter.)
According to iNews, NHS England recorded its worst cancer treatment waiting times on record in July 2018, with more than 3,000 people waiting longer than two months for cancer treatment to begin. NHS’ target is for 85% of patients to begin cancer treatment within 62 days of being referred by their general practitioner.
Anatomic pathologists in the United States should consider how the UK’s average delay in starting cancer treatment affects patients in that country. It is a metric that patients in the US would not tolerate. However, in the UK’s single payer system, the government’s National Health Service sets the budgets for training new physicians, including histopathologists. For many years, the pathology profession in the UK has regularly advocated for increasing the number of histopathologists trained each year by the medical schools in that country.
In July, the number of patients waiting for treatment longer than 60 days fell to 78.2%, the 31st month in a row the target was breached, iNews reported.
“We know that histopathology consultant shortages contribute to at least part of that problem,” Martin told iNews.
The RCPath report highlights the intense pressures on histopathologists—most of whom working in understaffed laboratories—face from increased workloads, as new NHS cancer screening initiatives, an aging population, and a shift toward precision medicine result in a rising number of cases being referred to labs.
“Demand for pathology services has grown significantly in recent years and continues to grow,” Martin noted in the RCPath statement. “The pathology workforce has not increased in line with this demand. If this trend continues unchecked, clinical services could be in jeopardy. Making sure pathology services can cope with current and future demand is essential if we are to ensure early diagnosis and improve outcomes for patients.”
Lack/Loss of Trained Histopathologists an Ongoing Problem
This is not the first time the alarm has been sounded in the UK over the lack of investment in trained pathologists along with a growing shortage of trained professional staff. In 2017, Dark Daily reported on calls by pathologists and other physicians for the UK government to increase funding for trained medical laboratory professionals to avert a predicted critical shortage in laboratory staffing within the next decade. (See “Pathologists and Physicians in United Kingdom Comment on How Shortage of Medical Laboratory Professionals Could Soon Delay Essential Diagnostic, Therapeutic Testing,” February 6, 2017.)
In its most recent workforce report, The Royal College of Pathologists is reiterating its call for:
- Increased funding for training;
- Better information technology (IT) for day-to-day work;
- Capital investment to implement digital pathology more widely; and,
- Development of advanced clinical practitioner apprenticeships to help more biomedical scientists become independent practitioners who would work alongside medically-qualified histopathologists.
Long-term, the organization is calling for additional training slots for pathologists in universities as well as other changes to draw more scientists into the field.
Aging Pathology Staff Means Shortages in US a Possibility
The NHS has stopped short of declaring the pathologist shortage a crisis. Instead, a Department of Health and Social Care spokesperson in an interview with the BBC highlighted recent initiatives taken in response to the shortage. “There are hundreds more pathologists in the NHS compared to 2010 and hospitals have reduced spending on temporary agency staff by over half a billion pounds in the last year,” the spokesperson noted. “We are listening to staff, encouraging more flexible working, and have increased medical training places for home-grown doctors by 25%, to ensure the NHS has the workforce it needs for the future.”
Despite those steps, the NHS may have more work to do. According to the Royal College of Pathologists workplace survey, a quarter of all histopathologists in the UK are 55 or older, adding an approaching retirement crisis to the existing shortage.
US anatomic pathology groups and clinical laboratories should monitor these events with a keen eye. The American pathology industry is challenged by many of the same trends, such as an aging workforce and lackluster replacement initiatives. Time will tell if the US learns from the UK’s experience.
—Andrea Downing Peck
Related Information:
College Report Finds Severe Staff Shortages Across Services Vital to Cancer Diagnosis
Pathologist Shortage ‘Delaying Cancer Diagnosis’
Cancer Treatment Waiting Times Worsening Amid Shortage of Pathology Staff
Histopathology Staff Shortages ‘Affecting Cancer Diagnoses’
Cancer Waiting Times at Their Worst Ever Level
Meeting Pathology Demand
Pathology Staff Shortages Causing Delays to Cancer Diagnosis, Says Report
Pathologists and Physicians in United Kingdom Comment on How Shortage of Medical Laboratory Professionals Could Soon Delay Essential Diagnostic, Therapeutic Testing
Oct 29, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory Pathology, Laboratory Testing
900,000 Australians have opted out of the nation’s new digital electronic health record system due to privacy and security concerns plaguing the My Health Record database
Countries around the world continue to attempt creating a single national electronic health record (EHR) system. And though billions have been spent, success remains elusive. Australia (AU) also has joined the club of nations struggling to launch a shareable digital health record system. But though the country does have a national healthcare system, a significant number of Aussies have declined to participate in a national EHR system as well.
Privacy and Security the Biggest Challenge
In February, Dark Daily reported that Australia’s largest pathology laboratories signed agreements with the Australian Digital Health Agency (ADHA) to join the nationwide EHR project. And that, though they praised the potential of the AU’s My Health Record, a doctors’ advocacy organization also voiced concerns about functionality, interoperability, and added burden placed on providers.
My Health Record is a 16-year $2 billion (AU) project to create a digital database that contains the medical health records for nearly all of the country’s 24.7-million citizens. But the system’s rollout has been far from smooth. As of September 12, roughly 900,000 Australians had opted out of the program, which has been plagued by privacy and security concerns, ZDNet reported.
The developments in Australia concerning the effort to implement a single electronic health records system for patients are useful for those pathologists and medical laboratory managers who want to position their labs to support services like these. Australia is not the only country that faces challenges in the implementation of a single, nationwide EHR.
“Even though most Australians will likely end up having a My Health Record by the end of the year, it doesn’t mean the government can declare victory by any means,” wrote Contributing Editor Robert Charette in IEEE Spectrum. “Its e-health record system must quickly prove more beneficial and easier to use for healthcare practitioners and individuals than is currently the case, while avoiding any significant data breaches or privacy leaks. Otherwise, it will continue its past history of being ignominiously ignored until the system eventually suffers a slow, and very costly, death.”
Flawed and Unsecure
Technical glitches marred the mid-July start of the government’s opt-out period, but the biggest issues facing My Health Record are its ongoing privacy and security difficulties. The Australian Privacy Foundation (APF) argues My Health Record is a flawed “summary system” that offers minimal value to consumers or healthcare providers while exposing Australians to potential security breaches.
“The risks to your privacy, confidentiality, and information security need to be balanced by the value of any of your health records,” APF states on its website. “In our assessment, because it is not really your health record but a less-reliable copy, the My Health Record has little value for either your clinicians or you as a patient: you both need the real thing. This means the risks to you may be high enough to question whether My Health Record is worth it.”
Not only have privacy advocates questioned My Health Record’s threat to information security and confidentiality, the former head of the federal agency tasked with building the system also has questioned the security of the online system.

Paul Shetler, a citizen of the UK and former Chief Digital Officer of the AU government’s Digital Transformation Agency (DTA), told the Australian Broadcasting Corporation he would “probably” withdraw from My Health Record if he were an Australian citizen. One of the main issues is My Health Record’s privacy settings automatically provide general access to all information within the record unless consumers take the time to set access codes to restrict access. (Photo copyright: diginomica.)
Stuck in a Time Warp
My Health Record does provide consumers with the ability to set controls that restrict access to their records. However, according to another ZDNet report, of the 971,252 records created during the EHR’s trial period, only 214 access controls were set. Of that number:
- 196 records had a code applied to the entire record;
- 10 had individual documents locked down with a code; and,
- eight had both record and document codes applied.
Grahame Grieve, Principal at Health Intersections, argues My Health Record is stuck in a time warp—a system built on technology that was state of the art in 2007—but that has not kept pace with technological advances during its years of development.
“In the last decade, there’s been a lot of change, smart phones, etc., and we’re all used to the way the Web works: a set of federated systems that act together to serve us,” he wrote in a statement to the Australian Senate committee tasked with investigating issues with My Health Record. “But the My Health Record is still frozen as if all this hasn’t happened: inconvenient, inflexible, with poorly controlled information access rules … Australia is lagging behind other countries which are prototyping innovative digital approaches to solve healthcare problems.”
According to IEEE Spectrum, the “political firestorm” that greeted the program’s nationwide launch caused the Australian Digital Health Agency to extend the opt-out period an extra month, to November 15, 2018. Doing so buys the government more time to pass legislation aimed at fixing other issues related to the 2012 My Health Record legislation, ZDNet noted.
In July, ZDNet reported My Health Record legislation would be amended to strengthen privacy provisions to ensure no health record can be released to police or government agencies, for any purpose, without a court order, and that those who cancel their My Health Record will have their record permanently deleted from the system.
The Australian Healthcare and Hospital Association (AHHA) and other healthcare associations have praised the e-Health initiative and urged consumers to opt-in to the system.
“The advantages of having your medical history in the one place, both for consumers and healthcare providers, are numerous,” then-AHHA Acting Chief Executive Linc Thurecht claimed in an AHHA statement. “Apart from convenience, the potential benefits include better coordination of care among multiple healthcare providers, better informed decisions on healthcare that involve both the patient and the healthcare provider, reduced duplication of diagnostic tests, fewer adverse drug events, and reduced hospital admissions.”
Other Failed National Health Systems
Australia, however, is not alone in hitting e-Health speedbumps. The United Kingdom in 2016 pulled the plug on the National Health Services’ care.data initiative following a review into concerns over privacy, lack of informed consent, and the sharing of medical data with drug and insurance firms.
And in Singapore, the government recently halted plans to have all healthcare providers upload data to the new National Electronic Health Record System after hackers stole the personal info of 1.5 million SingHealth patients in the nation’s worst cyberattack.
Meanwhile, in the United States the debate over national healthcare and protected health information (PHI) security is ongoing, and lessons like these from around the globe illustrate that solutions are not yet on the horizon.
Finding answers to consumers’ legitimate privacy and security concerns will be increasingly important for medical laboratories that will be called on to deliver more value to healthcare networks, and be required to share patient data with other healthcare providers to do so.
—Andrea Downing Peck
Related Information:
900,000 Australians Opt-Out of My Health Record
My Health Record: Former Digital Transformation Head Raises Concerns About Security of Online System
Australians Say No Thanks to Electronic Health Records
My Health Record Access Controls Used on 214 Times in Million Record Trial
Opt-Out Period for My Health Record Officially Extended Until November 15
My Health Record Brings Many Benefits
Personal Info of 1.5M SingHealth Patients, Including PM Lee, Stolen in Singapore’s Worst Cyber Attack
Australia Moves Closer to Nationwide Electronic Health Record as Nation’s Leading Pathology Laboratories Join Initiative
Oct 26, 2018 | Digital Pathology, Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing
Future EHRs will focus on efficiency, machine learning, and cloud services—improving how physicians and medical laboratories interact with the systems to support precision medicine and streamlined workflows
When the next generation of electronic health record (EHR) systems reaches the market, they will have advanced features that include cloud-based services and the ability to collect data from and communicate with patients using mobile devices. These new developments will provide clinical laboratories and anatomic pathology groups with new opportunities to create value with their lab testing services.
Proposed Improvements and Key Trends
Experts with EHR developers Epic Systems, Allscripts, Accenture, and drchrono spoke recently with Healthcare IT News about future platform initiatives and trends they feel will shape their next generation of EHR offerings.
They include:
- Automation analytics and human-centered designs for increased efficiency and to help reduce physician burnout;
- Improved feature parity across mobile and computer EHR interfaces to provide patients, physicians, and medical laboratories with access to information across a range of technologies and locations;
- Integration of machine learning and predictive modeling to improve analytics and allow for better implementation of genomics-informed medicine and population health features; and
- A shift toward cloud-hosted EHR solutions with support for application programming interfaces (APIs) designed for specific healthcare facilities that reduce IT overhead and make EHR systems accessible to smaller practices and facilities.
Should these proposals move forward, future generations of EHR platforms could transform from simple data storage/retrieval systems into critical tools physicians and medical laboratories use to facilitate communications and support decision-making in real time.
And, cloud-based EHRs with access to clinical labs’ APIs could enable those laboratories to communicate with and receive data from EHR systems with greater efficiency. This would eliminate yet another bottleneck in the decision-making process, and help laboratories increase volumes and margins through reduced documentation and data management overhead.
Cloud-based EHRs and Potential Pitfalls
Cloud-based EHRs rely on cloud computing, where IT resources are shared among multiple entities over the Internet. Such EHRs are highly scalable and allow end users to save money by hiring third-party IT services, rather than maintaining expensive IT staff.
Kipp Webb, MD, provider practice lead and Chief Clinical Innovation Officer at Accenture told Healthcare IT News that several EHR vendors are only a few years out on releasing cloud-based inpatient/outpatient EHR systems capable of meeting the needs of full-service medical centers.
While such a system would mean existing health networks would not need private infrastructure and dedicate IT teams to manage EHR system operations, a major shift in how next-gen systems are deployed and maintained could lead to potential interoperability and data transmission concerns. At least in the short term.
Yet, the transition also could lead to improved flexibility and connectivity between health networks and data providers—such as clinical laboratories and pathologist groups. This would be achieved through application programming interfaces (APIs) that enable computer systems to talk to each other and exchange data much more efficiently.
“Perhaps one of the biggest ways having a fully cloud-based EHR will change the way we as an industry operate will be enabled API access.” Daniel Kivatinos, COO and founder of drchrono, told Healthcare IT News. “You will be able to add other partners into the mix that just weren’t available before when you have a local EHR install only.”

Paul Black, CEO of Allscripts, believes these changes will likely require more than upgrading existing software or hardware. “The industry needs an entirely new approach to the EHR,” he told Healthcare IT News. “We’re seeing a huge need for the EHR to be mobile, cloud-based, and comprehensive to streamline workflow and get smarter with every use.” (Photo copyright: Allscripts.)
Reducing Physician Burnout through Human-Centered Design
As Dark Daily reported last year, EHRs have been identified as contributing to physician burnout, increased dissatisfaction, and decreased face-to-face interactions with patients.
Combined with the increased automation, Carl Dvorak, President of Epic Systems, notes next-gen EHR changes hold the potential to streamline the communication of orders, laboratory testing data, and information relevant to patient care. They could help physicians reach treatment decisions faster and provide laboratories with more insight, so they can suggest appropriate testing pathways for each episode of care.
“[Automation analytics] holds the key to unlocking some of the secrets to physician well-being,” Dvorak told Healthcare IT News. “For example, we can avoid work being unnecessarily diverted to physicians when it could be better managed by others.”
Black echoes similar benefits, saying, “We believe using human-centered design will transform the way physicians experience and interact with technology, as well as improve provider wellness.”
Some might question the success of the first wave of EHR systems. Though primarily built to address healthcare reform requirements, these systems provided critical feedback and data to EHR developers focused not on simply fulfilling regulatory requirements, but on meeting the needs of patients and care providers as well.
If these next-generations systems can help improve the quality of data recording, storage, and transmission, while also reducing physician burnout, they will have come a long way from the early EHRs. For medical laboratory professionals, these changes will likely impact how orders are received and lab results are reported back to doctors in the future. Thus, it’s worth monitoring these developments.
—Jon Stone
Related Information:
Next-Gen EHRs: Epic, Allscripts and Others Reveal Future of Electronic Health Records
Next-Gen IT Infrastructure: A Nervous System Backed by Analytics and Context
EHR Systems Continue to Cause Burnout, Physician Dissatisfaction, and Decreased Face-to-Face Patient Care









