Sep 5, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory Pathology, Laboratory Testing
While approaches differ between the three companies, heavy investment in EMR/EHR and other HIT solutions could signal significant changes ahead for a market currently dominated by only a few major developers
If healthcare big data is truly a disruptive force in healthcare’s transformation, then a big battle looms for control of that data. Some experts say that the companies now dominating the electronic health record (EHR) market will soon face tough competition from the world’s biggest tech companies.
Until recently, most clinical laboratories, anatomic pathology groups, hospitals, and other healthcare providers have depended on EHR systems from just a handful of health information technology (HIT) developers. But tech giants Google, Apple, and Microsoft have been filing hundreds of HIT related patents since 2013 and appear poised to compete on a large scale for a chunk of the EMR/EHR/HIT market, according to coverage in EHR Intelligence of Kalorama Information’s “EMR 2018: The Market for Electronic Medical Records” report.
How this will impact medical laboratories and pathology practices remains to be seen. Labs are sure to be influenced by coming events, since clinical laboratory test data represents the largest proportion of an individual patient’s permanent medical record. It’s important to note, though, that while most EHR/HIT developers have been motivated by federal incentives, Google (NASDAQ:GOOG), Apple (NASDAQ:AAPL), and Microsoft (NASDAQ:MSFT) are motivated by consumer demand, which increasingly dictates the direction of health technology development.
Thus, they may be better positioned to compete moving forward, as patients, physicians, and hospitals turn to precision medicine and value-based care for improved outcomes and increased revenues.

“The EMR efforts have moved hospitals from paper to digital records,” Bruce Carlson (above), Publisher of Kalorama Information, told HIT Infrastructure. “The next step is for tech giants to glean the data and improve upon that infrastructure. We’ll be talking about EHR in different ways in the next ten years.” (Photo copyright: Twitter.)
EMR/EHR Market Poised for Disruption
According EHR Intelligence, as of 2017, 97% of all US non-federal acute care hospitals and 84% of US hospitals had adopted an EHR system. Of these hospitals, more than half (50.5%) use products from just two developers—Cerner or Epic. That’s according to Health Data Management’s coverage of the KLAS report “US Hospital EMR Market Share 2017.”
However, recent interest in HIT and EHR systems by major Silicon Valley tech companies could lead to potential disruptions in the current state of the market. According to The New York Times, in the first 11 months of 2017, 10 of the largest US technology companies were involved in healthcare equity deals worth $2.7-billion. This marks a drastic increase over the 2012 figure of $277-million.
Though each company is approaching the market differently, Google, Microsoft, and Apple are all working on projects that could influence how both consumers and healthcare professionals interact with and utilize medical record data.
Of the three, Apple is the most consumer-centric with their Apple Health personal health record (PHR) integration into Apple iOS for iPhones and iPads. Microsoft, however, is working on developing analytics tools and storage solutions aimed at healthcare providers in general. And Google, through its parent company Alphabet, is focusing on data processing and storage.
Amazon also is working on its own HIT project which it calls 1492. While details are scant, HIT Infrastructure reports that the project is focused on interoperability among disparate EHR systems to improve sharing of protected health information (PHI) between providers, patients, and other healthcare providers, such as clinical labs and pathology groups. HIT Infrastructure also reported on rumors of Amazon branching into telemedicine using their Amazon Echo and Alexa platforms.
Security Concerns and Opportunities for Clinical Laboratories
According to Computerworld’s coverage of IDC research, by 2020, 25% of patients are expected to be taking part in ‘bring your own data” healthcare scenarios. Tech-savvy medical laboratories could find opportunities to interact directly with patients and encourage follow-through on test orders or follow-up on routine testing.
However, shifting protected health information to devices carried by consumers is not without risks.
“How do I know the data won’t make its way to some cloud somewhere to be shared, sold, etc.” Jack Gold, Principal Analyst with J. Gold Associates, told Computerworld. “And if I rely on an app to tell me what to do—say, take my meds—and it somehow gets hacked, can it make me sick, or worse?”
These are important questions and developments, which Dark Daily has covered in other recent e-briefings. (See, “Apple Updates Its Mobile Health Apps, While Microsoft Shifts Its Focus to Artificial Intelligence. Both Will Transform Healthcare, But Which Will Impact Clinical Laboratories the Most?” July 25, 2018.)
Nevertheless, with tech giants already developing products for the consumer market and healthcare provider industry, it’s a given consumers will soon gain greater access to their own healthcare information. Whether patients will ultimately embrace it, how they will use it, and how developers will interact with the data, is still undefined. But it’s coming and clinical laboratories should be prepared.
—Jon Stone
Related Information:
Apple to Launch Health Records App with HL7’s FHIR Specifications at 12 Hospitals
How Google, Microsoft, Apple Are Impacting EHR Use in Healthcare
Microsoft, Apple, Google Secure HIT Infrastructure Patents
How Big Tech Is Going after Your Health Care
Amazon Secret Healthcare IT Tech Team Focuses on EHRs, Alexa
Apple’s Health Record API Released to Third-Party Developers; Is It Safe?
Apple, Cerner and Microsoft Are Interested in Buying AthenaHealth: Here’s Why This CEO Says They Won’t
Apple Says iOS Health Records Has over 75 Backers, Uses Open Standards
Report: Health Systems Share Apple Health Records Feedback
Apple Is Officially in the EHR Business. Now What?
Why Apple’s Move on Medical Records Marks a Tectonic Shift
Slideshow Where the Top 8 EMRs Are Deployed
Apple Updates Its Mobile Health Apps, While Microsoft Shifts Its Focus to Artificial Intelligence. Both Will Transform Healthcare, but Which Will Impact Clinical Laboratories the Most?
Apple’s Update of Its Mobile Health App Consolidates Data from Multiple EHRs and Makes It Easier to Push Clinical Laboratory Data to Patients
Aug 31, 2018 | Digital Pathology, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
Affected patients speak about emotional, financial, and medical costs of receiving inaccurate results from the startup’s faulty Edison ‘finger-stick’ blood draw testing device
Healthcare consumers trust America’s clinical laboratories and anatomic pathology groups to provide accurate test results. When those test results are inaccurate, the loss of public trust can trigger a sharp decline in referrals/revenue and draw an avalanche of lawsuits by those harmed by inaccurate results.
The most recent example of this object lesson is disgraced blood testing company Theranos, previously estimated to be worth $9 billion but now struggling to stay afloat. The once high-flying startup has been brought to the edge of bankruptcy in the aftermath of a fraud settlement with the Securities and Exchange Commission (SEC), sanctions from the Centers for Medicare and Medicaid Services (CMS), investor lawsuits, consumer lawsuits, and a settlement with Walgreens over claims about Theranos’ Edison portable blood analyzer.
Theranos first made its unproven finger-stick blood draw device available to consumers in September 2013, when it announced a partnership with drugstore chain Walgreens (NASDAQ:WBA). At its height, Theranos operated 40 “Wellness Centers” in Walgreens stores in Arizona and a single location in California, which were the source of much of its revenue. USA Today reported the metro Phoenix-area centers alone sold more than 1.5 million blood tests, which yielded 7.8 million tests results for nearly 176,000 consumers. Theranos shuttered the wellness centers in 2016 after CMS inspectors found safety issues at Theranos’ laboratories in California and a Wall Street Journal (WSJ) investigation raised questions about the company’s testing procedures and accuracy claims. Ultimately, Theranos voided the results of all blood tests run on its Edison device from 2014 through 2015.

Breast-cancer survivor Sheri Ackert (above) told the WSJ she panicked when blood-test results from Theranos indicated her cancer may have reoccurred or were indicative of a rare type of tumor. After being retested by a different clinical laboratory, her results were found to be normal. Click here to watch a WSJ video about Ackert’s experience. (Photo/video copyright: Mark Peterman/Adya Beasley/Wall Street Journal.)
USA Today outlined the impact Theranos’ supposedly low-cost, cutting-edge technology had on several customers:
- A woman inaccurately diagnosed with the thyroid condition Hashimoto’s disease changed her lifestyle, made unnecessary medical appointments, and took medication she didn’t need;
- A woman inaccurately diagnosed with the autoimmune disease Sjögren’s syndrome was checked for food allergies before being retested and found not to have an autoimmune condition; and,
- An Arizona resident who had heart surgery visited a Theranos clinic five times to monitor the results of blood-thinning drug warfarin and was switched to a different drug. He had to have a second heart surgery to drain blood from the pericardial sac and believes more accurate test results could have averted the follow-up operation.
Arizona resident Steven Hammons visited a Theranos clinic several times to have his blood tested. He’d been placed on blood thinners following heart surgery. He was taken off the blood thinners presumably based on the results of those tests. However, as USA Today reported, one test result was later found to be inaccurate. Hammons, who underwent a second procedure to remove blood that had built up around his heart, told USA Today he was concerned about the safety of his fellow citizens.
“That makes me very concerned and worried for the safety of other Arizonans,” said Hammons, who once worked in the medical services division of a private health insurance company. “Government had a role in patient safety. The powers that be dropped the ball.”
Arizona Attorney General Mark Brnovich spearheaded a lawsuit against Theranos under the state’s Consumer Fraud Act, which led to a $4.65 million settlement covering full refunds for every Arizona customer who used the company’s testing services.
“Theranos may have not only had some erroneous test results, but they may have misread my rising blood pressure level as well,” Brnovich told The Republic in a 2017 article announcing the state’s fraud settlement with Theranos. “They said that about 10% of the results were inaccurate. The problem is, as an Arizona consumer, you don’t know whether you were part of that class or not.”
Downfall of a Once-Vaunted Clinical Laboratory Company
Dark Daily and sister publication The Dark Report have written extensively about these events. Former CEO Elizabeth Holmes founded Theranos in 2003 when she was just 19-years old. By 2013, Holmes had become a media sensation based on her claims that “Theranos had developed a medical technology that could do what seemed to be impossible: Its secret machines could run thousands of medical tests using the blood from a tiny finger-prick, and do so quickly and cheaply,” Bloomberg reported in a recent article outlining Holmes’ fall from grace.
While Holmes continues in the role of Chairman of Theranos’ Board of Directors, she was stripped of control of the company as part of the SEC settlement in 2016. The SEC found Holmes and then-company President Ramesh “Sunny” Balwani had fabricated claims Theranos technology had been validated by the Food and Drug Administration (FDA) and pharmaceutical companies and battle-tested by the US military in Afghanistan.
As a result, the SEC also barred Holmes from serving as an officer or director of any public company for 10 years. In October 2016, Theranos announced it would be closing its laboratory operations and focusing on its effort to create miniature medical testing machines, which it did. Nevertheless, the fallout continues.
As pressures on medical laboratories and pathology groups to cut costs while delivering quality care and value increases, laboratory leaders must not lose sight of the fact that accuracy of results remains the key to maintaining trust with healthcare consumers and a financially viable business.
—Andrea Downing Peck
Related Information:
Theranos, CEO Holmes, and Former President Balwani Charged with Massive Fraud
Theranos Receives Notice of Sanctions from the Centers for Medicare & Medicaid Services
Two More Investors Sue Theranos and Elizabeth Holmes for Fraud
Theranos Hit with Consumer Lawsuit over Faulty Blood Tests
Theranos, Walgreens Reportedly Reach a Deal to Settle Suit for under $30 Million
Theranos Selects Walgreens as a Long-Term Partner Through Which to Offer Its New Clinical Laboratory Service
An Open Letter from Elizabeth Holmes
How Startup Theranos Has Struggled with its Blood-Test Technology
Theranos Reaches $4.65 Million Fraud Settlement with Arizona
As Theranos Drama Unwinds, Former Patients Claim Inaccurate Tests Changed Their Lives
Theranos Statement on CMS 2567 Report
Agony, Alarm and Anger for People Hurt by Theranos’ Botched Blood Tests
Blood, Fraud and Money Led to Theranos CEO’s Fall from Grace
Holmes, Balwani Indicted by Department of Justice
Theranos News Gets Worse for the Former Silicon Valley Hero
After AACC Presentation, Elizabeth Holmes and Theranos Failed to Convince Clinical Laboratory Scientists and the News Media about Quality of Its Technology
Now Theranos Faces Criminal Investigation on Whether the Clinical Laboratory Company Misled Investors, according to Published Reports
Aug 27, 2018 | Laboratory Management and Operations, Laboratory News, Laboratory Operations, Management & Operations, News From Dark Daily
Data generated by medical laboratories and diagnostic providers takes an increasing role in treatment and precision medicine and allows greater analysis of data and integration of data into the care process
Most anatomic pathologists recognize that the unstructured data that makes up most pathology reports also represents a barrier to more sophisticated use of the information in those pathology reports. One solution is for pathology groups to adopt synoptic reporting as a way to get a pathology report’s essential data into structured fields.
The healthcare marketplace recognizes the value of structured data. In 2012, venture capitalists funded a new company called Flatiron Health. Flatiron’s goal was to access the medical records of cancer patients specifically to extract the relevant—and generally unstructured—data and put it into a structured database. This structured database could then be used to support both research and clinical care for cancer patients.
How valuable is structured healthcare data? Just this February, Roche paid $1.9 billion to acquire Flatiron. At that point, Flatiron had assembled information about the health records of two million cancer patients.
But Roche (ROG.S), recognizing the value of data, was not done. In July, it entered into an agreement to pay $2.4 billion for the remaining shares of cancer-testing company Foundation Medicine that it did not own. Foundation Medicine sequences tumors and uses that genetic data to assist physicians in diagnosing cancer, making treatment decisions, and identifying cancer patients who qualify for specific clinical trials.
Anatomic pathologists play a central role in the diagnosis, treatment, and monitoring of cancer patients. It behooves the pathology profession to recognize that generating, storing, analyzing, and reporting the data generated from examinations of tumor biopsies is a critical success factor moving forward. Otherwise, other players and stakeholders will move past the pathology profession and stake their own claim to capturing, owning, and using that data to add value in patient care.
How Lack of Standards Impact Transfer of Patient Data
DATAMARK Inc., a business process outsourcing (BPO) company headquartered in El Paso, Texas, reports that analysts from Merrill Lynch, Gartner, and IBM estimate unstructured data comprises roughly 80% of the information in the average electronic medical record. This data could be the key to improving outcomes, tailoring precision medicine treatments, or early diagnosis of chronic diseases.
From narrative descriptions of biopsies to dictated entries surrounding preventative care appointments, these entries hold data that might have value but are difficult to collate, organize, or analyze using software or reporting tools.
To further complicate matters, each service provider in a patient’s chain of care might hold different standards or preferred methods for recording data.
“At this point, [standards] are not to a level that helps with the detailed clinical data that we need for the scientific questions we want to ask,” Nikhil Wagle, MD, Assistant Professor of Medicine, Dana-Farber Cancer Institute, Harvard Medical School, and Associate Member, Broad Institute, told the New York Times.
An oncologist at the Dana Farber Cancer Institute in Boston, Wagle and his colleagues are creating a database of metastatic breast cancer patients capable of linking medical records, treatments, and outcomes with their genetic backgrounds and the genetics of their tumors. Despite best efforts, they’ve only collected 450 records for 375 patients in 2.5 years.

Nikhil Wagle, MD (above), Assistant Professor of Medicine, Dana-Farber Cancer Institute, Harvard Medical School, and Associate Member, Broad Institute, is building databases that link patient outcomes and experiences with their EHRs. But sharing that information has proved problematic, he told the New York Times. “Patients are incredibly engaged and excited,” he said, “[But] right now there isn’t a good solution. Even though the patients are saying, ‘I have consented for you to obtain my medical records,’ there is no good way to get them.” (Photo copyright: Dana-Farber Cancer Institute.)
Additionally, once records are obtained, the information—sometimes spanning hundreds of faxed pages—must still be processed into data compatible with Dana-Farber’s database. And updating and maintaining the database requires a full-time staff of experts that must review the information and accurately enter it as required.
When critical concerns arise—such as a cancer diagnosis—information that could yield valuable clues about treatment options and improve outcomes might be held in any number of data silos in any number of formats.
This doesn’t account for the complexity of organizing such information for researchers who are developing new treatments, applying data to less targeted approaches, or dealing with privacy concerns between care providers.
Moving forward, those who can create and interact with data in a way that requires minimal human touch to make it suitable for analysis, further processing, or archiving, could communicate data more effectively and glean value from the growing trove of data silos created by laboratories around the world.
Big Pharma Making Big Bets on Structured Data
These are all the reasons why the recent moves by Roche show the importance and perceived value of structured medical records data as it takes an increasingly important role in precision medicine treatments and diagnosis.
With its acquisition of both Flatiron Health and Foundation Medicine, Roche has secured the ability to generate data, convert said data into a structured format to drive decisions, improve core data-related services, and promote the value of their offerings. This positions Roche to maximize the value of its data for internal use and marketing to researchers and other interested parties.
For clinical laboratories, pathology groups, and other diagnostics providers generating untold amounts of data daily, this highlights a critical opportunity to stay ahead of future trends and position themselves as valuable sources of information as healthcare data continues to play an essential role in modern healthcare.
—Jon Stone
Related Information:
New Cancer Treatments Lie Hidden under Mountains of Paperwork
Unstructured Data in Electronic Health Record Systems: Challenges and Solutions
Pharma Giant Roche Just Made a $2.4 Billion Bet on Cancer Data
Roche to Buy Flatiron Health for $1.9 Billion to Expand Cancer Care Portfolio
Why Drug Giant Roche’s $1.9 Billion Deal to Buy Data Startup Flatiron Health Matters
Roche Acquires the Outstanding Shares of Foundation Medicine for $2.4Bn
New Solutions for Unstructured Data May Help with Clinical Laboratory and Anatomic Pathology Data
Aug 24, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing
CDC reports more than 93-million US adults are obese, and health issues related to obesity include heart disease, stroke, type 2 diabetes, and cancers
In recent years, the role of the human microbiome in weight loss or weight gain has been studied by different research groups. There is keen interest in this subject because of the high rates of obesity, and diagnostic companies know that development of a clinical laboratory test that could assess how an individual’s microbiome affects his/her weight would be a high-demand test.
This is true of a study published this year in Mayo Clinic Proceedings. Researchers at Mayo Clinic looked at obese patients who were in an active lifestyle intervention program designed to help them lose weight. It was determined that gut microbiota can have a role in both hindering weight loss and supporting weight loss.
Gut Microbiota More Complicated than Previously Thought
The Mayo researchers determined “an increased abundance of Phascolarctobacterium was associated with [successful weight loss]. In contrast, an increased abundance of Dialister and of genes encoding gut microbial carbohydrate-active enzymes was associated with failure to [lose] body weight. A gut microbiota with increased capability for carbohydrate metabolism appears to be associated with decreased weight loss in overweight and obese patients undergoing a lifestyle intervention program.”
How do bacteria impede weight loss? Vandana Nehra, MD, Mayo Clinic Gastroenterologist and co-senior author of the study, explained in a news release.
“Gut bacteria have the capacity to break down complex food particles, which provides us with additional energy. And this is normally is good for us,” she says. “However, for some individuals trying to lose weight, this process may become a hindrance.”
Put another away: people who more effectively metabolized carbohydrates were the ones who struggled to drop the pounds, New Atlas pointed out.

Vandana Nehra, MD (left), and Purna Kashyap, MBBS (right), are Mayo Clinic Gastroenterologists and co-senior authors of the Mayo study. “While we need to replicate these findings in a bigger study, we now have an important direction to pursue in terms of potentially providing more individualized strategies for people who struggle with obesity,” Nehra noted in the news release. Thus, precision medicine therapy for obese individuals could be based on Mayo Clinic’s research. (Photo copyright: Mayo Clinic.)
Mayo Study Provides Clues to Microbiota Potential in Weight Loss
The Mayo researchers wanted to know how gut bacteria behave in people who are trying to lose weight.
They recruited 26 people, ranging in age from 18 to 65, from the Mayo Clinic Obesity Treatment Research Program. Fecal stool samples, for researchers’ analysis, were collected from participants at the start of the three-month study period and at the end. The definition of successful weight loss was at least 5% of body weight.
Researchers found the following, according Live Science:
- 2 lbs. lost, on average, among all participants;
- Nine people were successful, losing an average of 17.4 lbs.;
- 17 people did not meet the goal, losing on average just 3.3 lbs.; and,
- More gut bacterial genes that break down carbohydrates were found in stool samples of the unsuccessful weight loss group, as compared to the successful dieters.
The researchers concluded that “An increased abundance of microbial genes encoding carbohydrate-active enzyme pathways and a decreased abundance of Phascolarctobacterium in the gut microbiota of obese and overweight individuals are associated with failure to lose at least 5% weight following a 3-month comprehensive lifestyle intervention program.”
Purna Kashyap, MBBS, Mayo Clinic Gastroenterologist and co-senior author of the study, told Live Science, “The study suggests there is a need to take the microbiome into account in clinical studies (on weight loss), and it also provides an important direction to pursue in terms of providing individualized care in obesity.” The very basis of precision medicine.
Future Weight-Loss Plans Based on Patient’s Microbiota
The Mayo Clinic researchers acknowledged the small sample size and need for more studies with larger samples over a longer time period. They also noted in their paper that Dialister has been associated with oral infections, such as gingivitis, and its role in energy expenditure and metabolism is unclear.
Still, the study suggests that it may soon be possible to give people individualized weight loss plans based on their gut bacteria. Clinical laboratory professionals and pathologists will want to stay abreast of follow-up studies and replication of findings by other research teams. A future medical laboratory test to analyze patients’ microbiomes could help obese people worldwide as well as lab business volume.
—Donna Marie Pocius
Related Information:
Gut Microbial Carbohydrate Metabolism Hinders Weight Loss in Overweight Adults Undergoing Lifestyle Intervention with a Volumetric Diet
Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice
CDC: Adult Obesity Facts
Makeup of an Individual’s Gut Bacteria May Play Role in Weight Loss, Mayo Study Suggests
Struggle to Lose Weight? Your gut Bacteria May Be to Blame
Your Gut Bacteria May Make It Harder to Lose Weight
Diet Hit a Snag? Your Gut Bacteria May be Partly to Blame
Can’t Lose Weight? Your Gut Bacteria Could be to Blame, According to Study
Richness of Human Gut Microbiome Correlates with Metabolic Markers
Annual Medical Spending Attributable to Obesity: Payer- and Service-Specific Estimates
5 Ways Gut Bacteria Affect Your Health
Cornell Researchers Identify Gut Microbes That May Help Some People Remain Thin; Findings Could Result in Clinical Laboratory Tests to Analyze Microbiomes of Individuals
Clinical Laboratories Might Soon be Diagnosing Obesity and Guiding Therapies that Utilize Engineered Microbes
Aug 23, 2018 | Digital Pathology, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
Genetic testing, gene sequencing done by clinical laboratories and anatomic pathology groups underpin how first-mover hospitals, health networks are improving patient outcomes
In just a few weeks, an unprecedented gathering will bring together the nation’s most prominent first-mover health networks, hospitals, and companies operating programs that deliver precision medicine daily to patients in clinical care settings.
On Sept. 12-13, 2018, “Breakthroughs with Genetic and Precision Medicine: What All Health Network CEOs Need to Know,” will take place at the Hutton Hotel in Nashville, Tenn. “What differentiates these sessions is the emphasis on each organization’s strategy, how it launched its precision medicine programs, what is improving in patient outcomes, and how payers are reimbursing for these services,” stated Robert L. Michel, Executive Director of the Precision Medicine Institute in Austin, Texas. “This is not about the science of precision medicine. Rather, it is about the practical elements required for any hospital, health system, or physician group to actually set up and deliver a precision medicine service to patients on a daily basis.”
Precision Medicine’s First-Mover Hospitals and Providers to Speak
Health systems and hospitals headlining this special conference are:
Companies scheduled to present include:
- Illumina;
- Humana;
- Sonic Healthcare USA;
- Genome Medical;
- CQuentia, and,
- S. HealthTek.
Exhibitors include the above, plus: Thermo Fisher, Philips, Sunquest, and MyGenetx.
“This meeting will give you the insider’s understanding about delivering precision medicine in real patient care settings that cannot be accessed at other venues,” noted Michel. “The goal is to have first-mover providers share their experiences, thus providing a road map that other hospitals, physician practices, and other providers at this conference can take back and follow with confidence.”
Michel said that sessions will be dedicated to precision medicine strategies, how it is being used in oncology, primary care, the role of pharmacogenomics, and use of healthcare big data. Speakers will describe the clever ways innovative health networks and hospitals are using healthcare big data to inform physicians in ways that improve outcomes, lower the cost of care and, in two real-world case studies, are generating seven-figure reimbursement from shared savings programs with certain health plans.

This year’s keynote address is by Jeffrey R. Balser, MD, PhD (above), President and CEO, Vanderbilt University Medical Center and Dean of the Vanderbilt University School of Medicine, one of the most progressive and innovative health systems in the country. (Photo copyright: Vanderbilt University.)
Using Healthcare Big Data to Achieve Precision Medicine Success, Shared Savings
“Shared savings successes will be one of the breakthrough achievements reported at the Nashville event,” he explained. “We’ve invited two prominent provider organizations to share how they are using healthcare big data to support physicians in achieving improved patient outcomes while at the same time impressively reducing the overall cost of care. To my knowledge, this is the first time these precision medicine case studies have been presented at a national meeting.”
One such presentation will be delivered by Philip Chen, MD, PhD, Chief Healthcare Informatics Officer at Sonic Healthcare USA Austin, Texas. Their precision medicine goal was to use healthcare big data to help physicians better manage diabetes and other chronic conditions in their practices. This program involved a large primary care practice and a major health insurer. Now in its fourth year, Sonic Healthcare USA is earning six- and seven-figure payments as part of a shared savings arrangement with the insurer.
“Shared savings is definitely a Holy Grail for all large health networks and physician groups as payers drop fee-for-service and switch providers to value-based payments,” said Michel. “The experience of Sonic Healthcare in this innovative three-way collaboration with an insurer and a very large physician group demonstrates that a strong data analytics capability and engagement with physicians can simultaneously bend the cost-of-care-curve downward while improving patient outcomes, as measured year-by-year. This is a presentation every C-Suite executive should attend.
Strategic, Business, Operational, and Financial Aspects of Precision Medicine
“This conference—centered upon the strategic, business, operational, and financial aspects of a precision medicine program—came to be because it is the unmet need of every health network CEO and C-Suite administrator,” observed Michel. “Every healthcare leader tasked with developing an effective clinical and financial strategy for his or her institution knows that the real challenge in launching a precision medicine program for patient care is not the science.
“Rather, the true challenges come from how to support clinical needs with the availability of capital, recruiting experienced clinicians, and putting the right informatics capabilities in place,” he stated. “Most hospital and health network administrators recognize the risk of launching a precision medicine program too early. They know such programs can suck up huge amounts of resources without producing significant improvements in patient care. What adds to the risk is that payers may be slow to reimburse for precision medicine.”
Register today to guarantee your place at “Breakthroughs with Genetic and Precision Medicine: What All Health Network CEOs Need to Know,” (or copy and paste this URL in your browser: https://dark.regfox.com/precision-medicine-institute).
Register by September 1 and save $300 on tuition! Plus, take advantage of our special Team Discount Program, so you and your key team members can get the most out of the conference by attending together.
“Breakthroughs with Genetic and Precision Medicine: What All Health Network CEOs Need to Know” is the gold-standard summit for everyone active or interested in succeeding with precision medicine programs. Don’t miss out—register today!
—Michael McBride
Related Information:
Breakthroughs with Genetic and Precision Medicine: What All Health Network CEOs Need to Know—Full Agenda and Details
Breakthroughs with Genetic and Precision Medicine: What All Health Network CEOs Need to Know—Registration information
Ongoing Growth in Consumer Genetic Testing Pressures Hospitals, Healthcare Networks to Educate and Prepare Physicians
Syapse Creates Precision Medicine Council That Quickly Attracted 200 of the Biggest Hospitals and Health Networks as Members
When Ramping Up Genomic Programs, Health Network/Hospital CEOs and Executives Must Consider Emerging Technologies, Swiftly Rising Consumer Demand
Precision Medicine Success Hinges on Diagnostics’ Clinical Utility
Precision Medicine and Sharing Medical Data in Real Time: Opportunities and Barriers
Ongoing Growth in Volume of Clinical Laboratory Tests That Support Precision Medicine Due to Physician Acceptance; Payers Still Have Concerns
Aug 20, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
“On-a-chip” devices continue to advance and medical laboratories will be natural repositories for patient data as the technology continues to improve
Dark Daily has predicted that the future of clinical laboratory testing will include highly complex multi-analyte test panels. The biomarkers, however, could number in the hundreds or thousands. So, it’s interesting to see new research by a Massachusetts Institute of Technology (MIT) team currently developing a multi-biomarker organ test device for clinical purposes.
Motivated by the costly failure of animal testing efforts to develop drug safety and efficacy in humans, the MIT research engineers created a microfluidic platform technology they dubbed “physiome-on-a-chip,” or more colloquially, “body-on-a-chip.” Their goal is to identify drug reaction in different cell groups within the body (in vivo).
They acknowledged contributions of in vitro microphysiological systems (MPSs), AKA “organ-on-a-chip” (OOC) systems. They note, however, in their paper published in Scientific Reports, that more complex systems that interconnect and receive data from multiple MPSs are needed due to increasing limitations arising from drugs’ “lack of efficacy” rather than toxicity.
“Here we describe the development and implementation of multi-MPS platforms, AKA physiome-on-a-chip, supporting four-way, seven-way, and 10-way MPS interactions for several weeks,” the MIT engineers wrote.
Though MIT’s new technology needs further research and development time, as well as clinical trials, this type of chip design and its ability to scale is a positive development and progress toward Dark Daily’s prediction. Once finalized, it could be adopted in medical laboratories for many types of diagnostic testing purposes.
Researchers Motivated to Improve Drug Efficacy
According to an MIT news release, “MIT engineers have developed new technology that could be used to evaluate new drugs and detect possible side effects before the drugs are tested in humans. Using a microfluidic platform that connects engineered tissues from up to 10 organs, the researchers can accurately replicate human organ interactions for weeks at a time, allowing them to measure the effects of drugs on different parts of the body.”
The “body-on-a-chip” technology, MIT says, is aimed at determining how drugs may affect one organ while also having side effects on others.
“Some of these effects are really hard to predict from animal models because the situations that lead to them are idiosyncratic. With our chip, you can distribute a drug and then look for the effects on other tissues and measure the exposure and how it is metabolized,” said Linda Griffith, PhD, Professor of Teaching Innovation at MIT’s School of Engineering, and a senior author of the study, in the news release.
According to MIT, factors affecting the effectiveness of pharmaceuticals may include:
- Genetics;
- Environment;
- Personal lifestyles; and,
- Interactions with other drugs.
TechCrunch called the study “unprecedented,” pointing to the platform’s connection of so many tissues and the technology’s ability to keep them stable for weeks.

“An advantage of our platform is that we can scale it up or down and accommodate a lot of different configurations,” Linda Griffith, PhD, MIT Professor, MIT School of Engineering, told Science Daily. “I think the field is going to go through a transition where we start to get more information out of a three-organ or four-organ system, and it will start to become cost-competitive because the information you’re getting is so much more valuable.” (Photo copyright: MacArthur Foundation.)
How “Body-on-a-Chip” Works
“Body-on-a-chip” is about the size of a tablet computer and links 10 organ types, including: liver, lung, gut, endometrium, brain, heart, pancreas, kidney, skin, and skeletal muscle.
Using microfluidic platform technology, the researchers placed one- to two-million cells from human tissue samples into the device and then pushed fluid through the chip to resemble blood flow, the Daily Mail reported, adding that MIT’s MPS platform design features:
- Compartments made from a plastic block;
- Passages for fluid to move (as a circulatory system does) between the compartments;
- A water reservoir to limit fluid evaporation; and,
- Ability to monitor flow of molecular exchanges and drug distribution.
Essentially, using the MIT device, a drug can be introduced to one organ, processed normally, and then passed to other organs for processing and use in other ways, TechCrunch summarized.
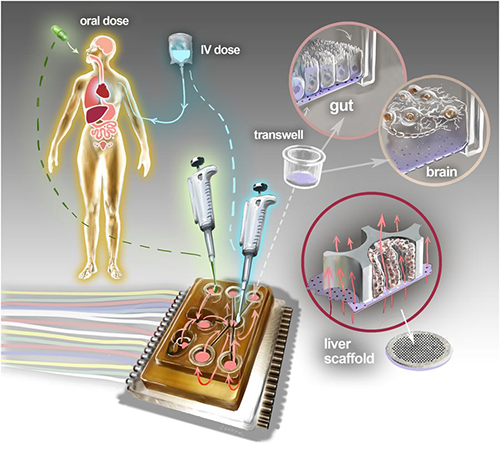
The physiome-on-a-chip system (above schematic) comprises bioengineered devices that nurture many interconnected 3D MPSs representing specified functional behaviors of each organ of interest, designed to capture essential features of in vivo physiology based on quantitative systems models tailored for individual applications such as drug fate or disease modeling. This technology could eventually be utilized for clinical laboratory and anatomic pathology testing. (Image and caption copyright: Victor O. Leshyk/Scientific Reports.)
Drug Delivery, Effects on Multiple Tissues Noted in MIT Study
The MIT researcher engineers reported these findings and accomplishments:
- Delivering a drug to the gastrointestinal tissue;
- Replicating digesting a drug;
- Observing as a drug was transported to other tissues and metabolized;
- Measuring a drug’s path; and,
- Noting effects of a drug on different tissues and how drugs break down.
“The huge potential of MPS technology is revealed by connecting multiple organ chips in an integrated system for in vitro pharmacology. This study beautifully illustrates that multi-MPS ‘physiome-on-a-chip’ approaches, which combine the genetic background of human cells with physiologically relevant tissue-to-media volumes, allow accurate prediction of drug pharmacokinetics and drug absorption, distribution, metabolism, and excretion,” said Kevin Healy, PhD, Professor of Bioengineering and Materials Science and Engineering, at University of California Berkeley in the MIT news release. Healy was not involved in the research.
Unique Device Design
In addition to making it possible to study so many different tissue types, the device design, according to MIT, is unique for these reasons:
- Its open microfluidic system, rather than a closed system, means the lid can be removed to manipulate tissue samples;
- Instead of external pumps common in closed systems, the MIT team used “on-board pumps” to control flow of liquid between the organs; and,
- The pumps used enabled larger engineered tissues, such as those from tumors in an organ, to be assessed.
The MIT engineers next plan to focus on specific organs—including the brain, liver, and gastrointestinal tissue—to model Parkinson’s disease, Digital Trends reported.
As healthcare providers and medical laboratories adopt precision medicine, MIT’s contributions are both timely and important. The ability to accommodate many different configurations in one platform is impressive, and something Dark Daily has been anticipating.
—Donna Marie Pocius
Related Information:
A “Body-on-a-Chip” Strings Together 10 Model Human Organs
“Body-on-a-Chip” Could Improve Drug Evaluation
MIT Builds “Body-on-a-Chip” Device That Can Store up to 10 Artificial Organs at Once
Interconnected Microphysiological Systems for Quantitative Biology and Pharmacology Studies
MIT Gadget Puts Multiple Artificial Organs into a Paperback-Sized Connected System
Drug Testing Could Get a Boost from MIT’s “Body-on-a-Chip”










