If the proposed rule becomes final, it may shift some inpatient medical laboratory testing away from hospital labs and to independent clinical laboratories
Medical laboratories in hospitals and health systems already feel the pinch of less test orders originating from their own emergency departments (ED). Now, more tests associated with inpatient care might also shift away from hospital labs due to a new proposed rule from the federal Centers for Medicaid and Medicare Services (CMS) that would move 1,740 specific procedures from inpatient care settings to outpatient ambulatory surgical centers (ACS).
Further, the proposed rule would completely phase out the “inpatient only” (IPO) list of services over a three-year transitional period, with total elimination of the IPO list by Calendar Year (CY) 2024.
If finalized as written, the rule (CMS-1736-P) would have a negative impact on the finances of hospitals laboratories as more patients get their care in outpatient settings instead of their local hospitals.
Conversely, hospital outreach labs that service ambulatory surgical centers and other outpatient settings could have an opportunity to pick up more medical laboratory test referrals.
The proposed rule, titled, “Medicare Program: Hospital Outpatient Prospective Payment and Ambulatory Surgical Center Payment Systems and Quality Reporting Programs; New Categories for Hospital Outpatient Department Prior Authorization Process; Clinical Laboratory Fee Schedule: Laboratory Date of Service Policy; Overall Hospital Quality Star Rating Methodology; and Physician-Owned Hospitals,” was published in the Federal Register on August 12, 2020, and is open for comments until 10/05/2020.
Its summary reads: “This proposed rule would revise the Medicare hospital outpatient prospective payment system (OPPS) and the Medicare ambulatory surgical center (ASC) payment system for Calendar Year (CY) 2021 based on our continuing experience with these systems.
“In this proposed rule, we describe the proposed changes to the amounts and factors used to determine the payment rates for Medicare services paid under the OPPS and those paid under the ASC payment system.
“Also, this proposed rule would update and refine the requirements for the Hospital Outpatient Quality Reporting (OQR) Program and the ASC Quality Reporting (ASCQR) Program. In addition, this proposed rule would establish and update the Overall Hospital Quality Star Rating beginning with the CY 2021; remove certain restrictions on the expansion of physician-owned hospitals that qualify as ‘high Medicaid facilities,’ and clarify that certain beds are counted toward a hospital’s baseline number of operating rooms, procedure rooms, and beds; and add two new service categories to the OPD [Outpatient Department] Prior Authorization Process.”
Moving from Highest Cost Settings to Lower Cost Settings
In the big picture, these changes can save Medicare money. By shifting procedures for Medicare patients from the highest cost settings—hospital inpatient—to lower cost settings, such as outpatient ambulatory surgical centers, and by eliminating the inpatient-only list, physicians have more leeway to determine for themselves whether a patient needs to be hospitalized for any given procedure.
In “Do Hospitals Have a Target on their Back?” healthcare coding and reimbursement consultant, Terry Fletcher, an editorial board member with ICD10monitor, wrote, “Last year, CMS proposed removing certain services from the inpatient-only list and making them available on an outpatient basis, which it said would help lower costs.
“According to the proposal, ambulatory surgical centers would get a payment increase of 2.6%, and CMS estimated total payments to them for 2021 will be about $5.45 billion, an increase of $160 million from this year,” she added.
Fewer Referrals for Inpatient Lab, More for Hospital Outreach Labs
The impact of the proposed rule is predictable—price shopping will ensue, which is what Medicare wants. Thus, with the removal of the inpatient-only procedure list, the clinical laboratories of hospitals and health systems will likely see a reduction in inpatient test orders. But clinical laboratories participating in hospital outreach programs may see an increase in test orders, as doctors transition to more outpatient procedures.
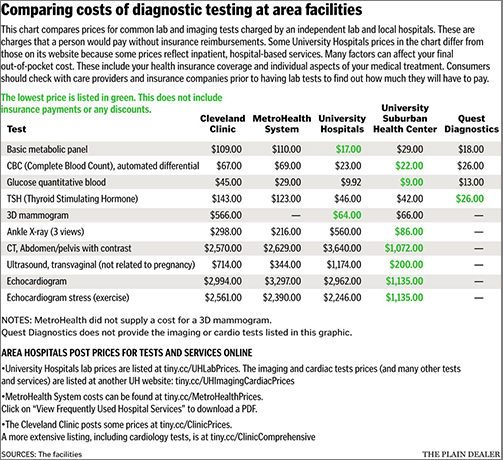
This seemingly simple shift may be more complicated than it appears, however, for both patients and labs. “In general, any routine test is going to be more expensive at a hospital,” Jean Pinder, founder and CEO of ClearHealthCosts, told Cleveland.com.
There may be other concerns as well. Convenience, insurance coverage, and physician recommendations often influence patient decisions about clinical laboratories.
Change Is the Only Constant
The entire healthcare industry is undergoing change that is unlikely to end any time soon. Clinical laboratory managers who stay aware of trends in the industry and remain informed on regulatory changes, and who look for opportunities as the business landscape evolves, will have the best chance for guiding their labs to success.
That would certainly be true if CMS is able to publish a final rule that shifts a large number of procedures away from inpatient care and categorizes them as outpatient procedures.
—Dava Stewart
Related Information:
CMS Proposed Rule (CMS-1736-P) Medicare Program
Do Hospitals Have a Target on their Back?
PAMA Price Reporting Update: Insights to Help Prepare to Meet the Requirement





This could be the straw that broke the camels back for a lot of hospitals. This change would make Quest Diagnostics and or Lab Corp monopolize the outpatient test revenue. Money that should stay in our small communities would leave and profit a large private lab. There must be some kickbacks from Quest and Lab Corp to make this happen.