Medical Laboratory Test Offers Improved Accuracy and Faster Time-To-Answer
It’s big news when an international health body endorses a new proprietary clinical laboratory test. That’s why pathologists will be interested to learn that, earlier this month, the World Health Organization (WHO) publicly recommended that nations incorporate a new rapid molecular test for tuberculosis into their disease testing programs.
The clinical laboratory test that the World Health Organization endorsed is the Xpert MTB/RIF test manufactured by Cepheid (Nasdaq: CPHD), the molecular diagnostics company based in Sunnyvale, California. The assay is a 100-minute rapid tuberculosis (TB) test. It is a fully-automated nucleic acid amplification test and WHO advised that the assay be introduced into clinical use under defined conditions as an integral part of a nation’s program to diagnose and treat TB and multi-drug resistant TB.
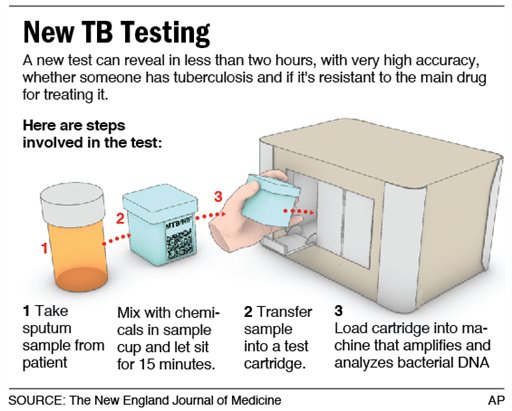
Here’s a diagram of the steps required to use Cepheid’s rapid molecular test for Tuberculosis. (From Associated Press, sourced from The New England Journal of Medicine.)
Molecular Pathology Test for TB Delivers More Clinical Value
Pathologists and clinical laboratory managers should consider the WHO’s acceptance of a proprietary medical laboratory test that incorporates new molecular technology as one more milestone on the road to molecular testing and genetic medicine. For developing countries, this new TB assay will make it possible to expand the locations where TB testing can be conducted while shortening the time-to-answer and producing a more accurate result.
Tuberculosis is a major problem in many countries. The WHO estimates that 2 billion people now have TB. Approximately 9 million more individuals become infected with TB annually, of which 2 million die of the disease. The WHO ranks TB among the world’s top 10 causes of death. Cepheid’s Xpert MTB/RIF test identifies both Mycobacterium Tuberculosis (MTB) and resistance to Rifampicin (RIF).
According to a Cepheid press release, “Rapid diagnosis of TB is vital in areas such as sub-Saharan Africa and Southeast Asia due to the close connection between HIV and TB. Sputum smear microscopy, which often delivers poor sensitivity in patients suffering from tuberculosis, is almost completely ineffective in those who also have HIV co-infection. The weakened immune system of an HIV-positive person is particularly susceptible to infection, resulting in one third of the 33 million HIV sufferers worldwide infected with TB. Left untreated, 90 percent of these people will die within months of first contracting the disease, reinforcing the urgent need for an accurate and rapid test.”
Disadvantages of Sputum Smear Microscopy in Testing for Tuberculosis
According to the WHO, “Many countries still rely principally on sputum smear microscopy, a diagnostic method that was developed over a century ago. But this new ‘while you wait’ test incorporates modern DNA technology that can be used outside of conventional laboratories. It also benefits from being fully automated and therefore easy and safe to use.”
In a recent Fox News report, Cepheid said it “would offer a 75% price discount for poorer nations on the tests and the table-top computer system used to analyze them, meaning the tests will cost $16.86 and the machine will cost around $17,000.”
“This preferential price would be granted to 116 low and middle-income countries where TB is endemic, and prices would be discounted further as the volume of demand rises in coming years,” said John Bishop, Cepheid’s CEO in the same report.
Cepheid developed the “while you wait” test in partnership with the Foundation for Innovative New Diagnostics (FIND), and the University of Medicine and Dentistry of New Jersey (UMDNJ). The National Institute of Allergy & Infectious Diseases (NIAID) funded the research.
“There has been a strong commitment to remove any obstacles, including financial barriers, that could prevent the successful roll-out of this new technology,” said FIND’s CEO Giorgio Roscigno. “For the first time in TB control, we are enabling access to state-of-the-art technology simultaneously in low, middle and high income countries,” he added.
“Clinicians will now be able to obtain dependable test results for not only detection of TB but simultaneous determination of whether or not it is a drug resistant strain in virtually any clinical setting,” said Bishop.
What particularly observant pathologists and clinical laboratory managers may have deduced from the comments presented above is the potential for this new rapid molecular TB test to find a place in the healthcare systems of developed countries. After all, developed countries have the same issues with the sensitivity and specificity of sputum smear microscopy when diagnosing tuberculosis and drug-resistant strains.
Related Information:
WHO Says Cepheid Rapid Test Will Transform TB Care




When the Gamma interferon tests were introduced 10 years ago they were deemed a very important development. Ten years later, and over 500 publications on evaluations, the tests are nothing better than a glorified mantoux test. Will the Cepheid Xpert MTB-Rif follow the same path. Already independent evaluations are showing that the NEJM data are not being reporduced at point of care. Time will tell the usefulness of Cepheid Xpert MTB-Rif -WHO should have responded with more caution
Excellent job,new molecular test for TB patients, sputum smear testing is being used with TB DOTS project in each endemic country, now with this new molecular technique and in less than 2 hours aacurate TB report is blessing for poor TB patients.
We wait to this new technology, when it will be implemented in resource poor settings, so that the lab staff will get rid from this old and inaccurate sputum testing.