May 7, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory Pathology, Laboratory Testing
Genomic analysis of pipes and sewers leading from the National Institutes of Health Clinical Care Center in Bethesda, Md., reveals the presence of carbapenem-resistant organisms; raises concern about the presence of multi-drug-resistant bacteria previously undetected in hospital settings
If hospitals and medical laboratories are battlegrounds, then microbiologists and clinical laboratory professionals are frontline soldiers in the ongoing fight against hospital-acquired infections (HAIs) and antibiotic resistance. These warriors, armed with advanced testing and diagnostic skills, bring expertise to antimicrobial stewardship programs that help block the spread of infectious disease. In this war, however, microbiologists and medical laboratory scientists (AKA, medical technologists) also often discover and identify new and potential strains of antibiotic resistance.
One such discovery involves a study published in mBio, a journal of the American Society for Microbiology (ASM), conducted by microbiologist Karen Frank, MD, PhD, D(AMBB), Chief of the Microbiology Service Department at the National Institutes of Health (NIH), and past-president of the Academy of Clinical Laboratory Physicians and Scientists (ACLPS). She and her colleagues identified a surprising source of carbapenem-resistant organisms—the plumbing, sewers, and wastewater beneath the National Institutes of Health Center (NIHCC) in Bethesda, Md. And they theorize similar “reservoirs” could exist beneath other healthcare centers as well.
Potential Source of Superbugs and Hospital-Acquired Infections
According to the mBio study, “Carbapenemase-producing organisms (CPOs) are a global concern because of the morbidity and mortality associated with these resistant Gram-negative bacteria. Horizontal plasmid transfer spreads the resistance mechanism to new bacteria, and understanding the plasmid ecology of the hospital environment can assist in the design of control strategies to prevent nosocomial infections.”

Karen Frank, MD, PhD (above), is Chief of the Microbiology Service Department at the National Institutes of Health and past-president of the Academy of Clinical Laboratory Physicians and Scientists. She suggests hospitals begin tracking the spread of the bacteria. “In the big picture, the concern is the spread of these resistant organisms worldwide, and some regions of the world are not tracking the spread of the hospital isolates.” (Photo copyright: National Institutes of Health.)
Frank’s team used Illumina’s MiSeq next-generation sequencer and single-molecule real-time (SMRT) sequencing paired with genome libraries, genomics viewers, and software to analyze the genomic DNA of more than 700 samples from the plumbing and sewers. They discovered a “potential environmental reservoir of mobile elements that may contribute to the spread of resistance genes, and increase the risk of antibiotic resistant ‘superbugs’ and difficult to treat hospital-acquired infections (HAIs).”
Genomic Sequencing Identifies Silent Threat Lurking in Sewers
Frank’s study was motivated by a 2011 outbreak of antibiotic-resistant Klebsiella pneumoniae bacteria that spread through the NIHCC via plumbing in ICU, ultimately resulting in the deaths of 11 patients. Although the hospital, like many others, had dedicated teams working to reduce environmental spread of infectious materials, overlooked sinks and pipes were eventually determined to be a disease vector.
In an NBC News report on Frank’s study, Amy Mathers, MD, Director of The Sink Lab at the University of Virginia, noted that sinks are often a locus of infection. In a study published in Applied and Environmental Microbiology, another journal of the ASM, Mathers noted that bacteria in drains form a difficult to clean biofilm that spreads to neighboring sinks through pipes. Mathers told NBC News that despite cleaning, “bacteria stayed adherent to the wall of the pipe” and even “splashed out” into the rooms with sink use.
During the 2011-2012 outbreak, David Henderson, MD, Deputy Director for Clinical Care at the NIHCC, told the LA Times of the increased need for surveillance, and predicted that clinical laboratory methods like genome sequencing “will become a critical tool for epidemiology in the future.”
Frank’s research fulfilled Henderson’s prediction and proved the importance of genomic sequencing and analysis in tracking new potential sources of infection. Frank’s team used the latest tools in genomic sequencing to identify and profile microbes found in locations ranging from internal plumbing and floor drains to sink traps and even external manhole covers outside the hospital proper. It is through that analysis that they identified the vast collection of CPOs thriving in hospital wastewater.
In an article, GenomeWeb quoted Frank’s study, noting that “Over two dozen carbapenemase gene-containing plasmids were identified in the samples considered” and CPOs turned up in nearly all 700 surveillance samples, including “all seven of the wastewater samples taken from the hospital’s intensive care unit pipes.” Although the hospital environment, including “high-touch surfaces,” remained free of similar CPOs, Frank’s team noted potential associations between patient and environmental isolates. GenomeWeb noted Frank’s findings that CPO levels were in “contrast to the low positivity rate in both the patient population and the patient-accessible environment” at NIHCC, but still held the potential for transmission to vulnerable patients.
Antibiotic-Resistance: A Global Concern
The Centers for Disease Control and Prevention (CDC) reports that more than two million illnesses and 23,000 deaths in the US are caused each year by antibiotic resistance, with 14,000 deaths alone linked to antibiotic resistance associated with Clostridium difficile infections (CDI). Worldwide those numbers are even higher.
Second only to CDI on the CDC’s categorized list of “18 drug-resistant threats to the United States” are carbapenem-resistant Enterobacteriaceae (CRE).
Since carbapenems are a “last resort” antibiotic for bacteria resistant to other antibiotics, the NIHCC “reservoir” of CPOs is a frightening discovery for physicians, clinical laboratory professionals, and the patients they serve.
The high CPO environment in NIHCC wastewater has the capability to spread resistance to bacteria even without the formal introduction of antibiotics. In an interview with Healthcare Finance News, Frank indicated that lateral gene transfer via plasmids was not only possible, but likely.
“The bacteria fight with each other and plasmids can carry genes that help them survive. As part of a complex bacterial community, they can transfer the plasmids carrying resistance genes to each other,” she noted. “That lateral gene transfer means bacteria can gain resistance, even without exposure to the antibiotics.”
The discovery of this new potential “reservoir” of CPOs may mean new focused genomic work for microbiologists and clinical laboratories. The knowledge gained by the discovery of CPOs in hospital waste water and sinks offers a new target for study and research that, as Frank concludes, will “benefit healthcare facilities worldwide” and “broaden our understanding of antimicrobial resistance genes in multi-drug resistant (MDR) bacteria in the environment and hospital settings.”
—Amanda Warren
Related Information:
Genomic Analysis of Hospital Plumbing Reveals Diverse Reservoir of Bacterial Plasmids Conferring Carbapenem Resistance
Snooping Around in Hospital Pipes, Scientists Find DNA That Fuels the Spread of Superbugs
CSI Bethesda: Sleuths Used Sequenced Genome to Track Down Killer
Antibiotic/Antimicrobial Resistance
Study Tracks How Superbugs Splash Out of Hospital Sink Drains
CDC: Biggest Threats
Antimicrobial Stewardship: How the Microbiology Laboratory Can Right the Ship
Superbugs Breeding in Hospital Plumbing Put Patients at Risk
Microbiologists at Weill Cornell Use Next-Generation Gene Sequencing to Map the Microbiome of New York City Subways
Jan 26, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Sales and Marketing, Laboratory Testing, Management & Operations
Lack of Medicare or third-party payer coverage for most genetic screening tests in healthy adults is not discouraging development of new gene testing products
With the global anatomic pathology genetic testing market poised to reach $9.8 billion by 2025, clinical laboratories continue to develop new genetic screening tests (rather than diagnostic tests) intended to help physicians identify patients who carry inherited genetic mutations that could put them or their future children at higher risk for chronic disease, such as cancer.
This is a bit of a gamble since (with some exceptions) Medicare and many health insurers typically will not pay for predictive and presymptomatic genetic tests and services used to detect an undiagnosed disease or disease predisposition.
Nevertheless, Inkwood Research of Gurugram, India, predicts in its “Global Genetic Testing Market Forecast 2017-2024” report that aging populations throughout the world will be the driving force producing “enormous opportunities for the global genetic testing market.” The research firm anticipates this will result in a 9.93% increase in annual sales revenue during each of the next seven years.
Screening versus Diagnostic Testing Gains Popularity Among Patients, Physicians
Genetic diagnostic testing promises to accelerate the growth of precision medicine by guiding the diagnosis and treatment of cancer and other chronic diseases. However, genetic tests that “screen” healthy patients for predispositions to certain diseases also are gaining traction in the marketplace.
The US Food and Drug Administration (FDA) gave direct-to-consumer genetic screening testing a boost in April 2017 when it allowed marketing of 23andMe Personal Genome Service Genetic Health Risk tests for 10 inherited diseases or conditions, including:
· Parkinson’s Disease;
· Late-onset Alzheimer’s Disease;
· Celiac Disease; and
· other conditions.
“Consumers can now have direct access to certain genetic risk information,” Jeffrey Shuren, MD, Director of the FDA’s Center for Devices and Radiological Health, said in a press release. “But it is important that people understand that genetic risk is just one piece of the bigger puzzle, it does not mean they will or won’t ultimately develop a disease.”
Robert Green, MD, MPH, a Professor of Medicine at Harvard Medical School, told NPR that consumers should have access to genetic information. However, they also need to understand its limitations.
“Some people really want this [genetic] information on their own, and others want it through their physician,” Green said. “Both those channels are legitimate. People should just be aware that this information is complicated.”
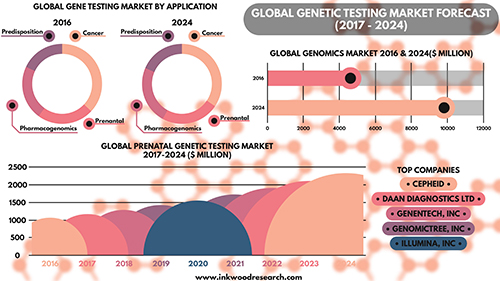
According to the Inkwood Research report, “The global genetic testing market is anticipated to grow from $4,614 million in 2016 to $9,806 million by 2025, at a CAGR [Compound Annual Growth Rate] of 9.93% between 2017 and 2025. The important driver increasing growth in the global genetic testing market is an aging population on the rise. The rising geriatric population is driving the global genetic testing market to a significant level.” (Caption and graphic copyright: Inkwood Research.)
· Cystic Fibrosis;
· Sickle Cell Disease; and
· Spinal Muscular Atrophy.
The genetic screening panel tests for the 22 heritable diseases cited by the American College of Obstetricians and Gynecologists (ACOG) in a Committee Opinion on genetic carrier screenings published by the ACOG in March 2017.
“The United States is truly a melting pot, and it no longer makes sense for physicians to assume genetic screening is appropriate for an individual based on presumed race or ethnicity,” Felicitas Lacbawan, MD, Executive Medical Director, Advanced Diagnostics, Quest Diagnostics, stated in a press release. “QHerit is designed for any woman and her partner, not just those in a specific, so-called high-risk ethnic or racial group.”
Genetic Screening in Primary Care Helps Assess Risk for Chronic Disease
Genetic diagnostic test developer Invitae (NYSE:NVTA) also points to growing evidence of the genetic screening test’s value to healthy individuals. In September 2017, Invitae presented initial findings at the National Society of Genetic Counselors 36th Annual Conference. The study showed a retrospective analysis of 120 patients tested with a proactive genetic screening panel for healthy adults had revealed medically significant findings for nearly one in five patients.
“Interest among otherwise healthy adults in using genetic information to understand their risk of disease conditions continues to grow each year, ” Robert Nussbaum, MD, Chief Medical Officer of Invitae, said in a press release. “These and other data show that interest is well-placed, with a substantial group of patients showing genetic variants associated with elevated risk of diseases like cancer where monitoring and early intervention can be helpful. Use of genetic screening in the primary care setting can assess risk to help shape individual screening plans. We are continually adding tools and resources that help reduce barriers to the widespread use of genetic information in mainstream medical practice.”
Routine Genetic Screening Could Become Norm, CDC Says
The Centers for Disease Control and Prevention (CDC) notes that newborn screening is “currently the largest public health genetics program in the world,” with more than four million babies screened at birth each year for 30 or more genetic conditions. In the CDC’s “Genomics and Health Impact Blog,” the agency continues to maintain a “cautionary attitude about personal genomic tests” beyond the newborn period, directing those considering direct-to-consumer laboratory testing, such as 23andMe and MyMedLab, to “think before you spit.”
Nonetheless, the CDC acknowledges routine genetic screening of healthy people could become the norm. However, others advise caution.
“To be sure, while the use of genome sequencing is promising in certain clinical scenarios, such as rare diseases and cancer, we do not think that whole genome sequencing in the general population is appropriate at this time,” Muin J. Khoury, PhD, MD, Director, Office of Public Health Genomics, CDC, wrote in a January 30, 2017, blog post. “We would not recommend its use outside research studies … But it is also becoming clearer that as science progresses, we are discovering more opportunities for using genetic screening of healthy individuals for preventing common diseases across the lifespan, outside of the newborn screening context.”
The impact on clinical laboratories and anatomic pathology groups should genetic screening become normalized should be clear: Labs will be tasked with performing these tests, and pathologists will be needed to interpret them and educate both physicians and patients on the findings.
Before that, however, genetic screening tests will need to be fully supported by government, and insurers, including Medicare, will have to agree to pay for them.
—Andrea Downing Peck
Related Information:
Global Genetic Testing Market Forecast 2017-2024
Carrier Screening for Genetic Conditions
Quest Diagnostics Launches QHerit, a Pan-Ethnic Genetic Screening Panel Aligned with New Medical Guidelines
Invitae Expands Test Menu for Proactive Genetic Testing in Healthy Adults
Invitae Highlighting New Research, Expanded Suite of Services at National Society of Genetic Counselors (NSGC) 36th Annual Conference
Consumer Genetic Testing: Think Before You Spit, 2017 Edition
Genetic Screening of Healthy Populations to Save Lives and Prevent Disease
FDA Allows Marketing of First Direct-to-Consumer Test that Provide Genetic Risk Information for Certain Conditions
FDA Approves Marketing of Consumer Genetic Tests for Some Conditions
Jan 23, 2018
WEBINARS UPCOMING WEBINARS FREE WebinarIs Mpox a Global Concern? Why Labs Need to Prepare for a New, Dangerous Cutaneous Strain Approaching the U.S. October 29, 2024 — SIGN UP HERE On August 14, 2024, the World Health Organization (WHO) declared mpox a global emergency once again. This latest outbreak is exacerbated by a more virulent strain known as clade 1b, which poses a significant threat to global communities. Every laboratory should proactively prepare for the potential arrival of this...

Jan 12, 2018 | Laboratory Pathology
Clinical labs and pathology groups know how advances in targeted therapies and genomics far outpace providers’ and patients’ ability to know how best to use and pay for them. One fascinating development on the road to precision medicine is that many new cancer drugs now in clinical trials will require a companion genetic test to identify patients with tumors that will respond to a specific therapeutic drug.
Sep 27, 2017 | Laboratory Pathology
High-powered hand dryers, like those used in public restrooms, are the latest targets in pursuit of cleanliness in public and medical environments
Microbiologists and clinical laboratory scientists will be fascinated by the findings of a research study into a method of hand drying that the study scientists described as like “virus hand grenades.” If these findings are confirmed by other studies, it may lead to changes in how hand washing stations in hospitals and medical laboratories are equipped, among other things.
Clinical laboratory personnel and pathology group members come into contact with, and fight against, biological contamination on a daily basis. Proper hand-washing/drying and waste disposal techniques, therefore, are critical functions for any well-run medical laboratory. That is why it is significant to learn that today’s most common hand-drying apparatus—the Jet Air Dryer—could be responsible for spreading infections germs through its everyday usage.
After studying hand-drying techniques, researchers at The University of Westminster in London determined that high-powered jet air dryers can act like “virus hand grenades.” The study, published in the Journal of Applied Microbiology earlier this year, compared the virus-spreading capabilities of three different types of hand-drying techniques:
1. Warm air dryers;
2. Jet air dryers; and
3. Paper towels.
To perform the research, participants placed MS2, an “icosahedral, positive-sense single-stranded RNA virus that infects the bacterium Escherichia coli and other members of the Enterobacteriaceae,” on their gloved hands. They then dried their hands using the various drying methods. Samples were collected around the three devices from different heights and distances on petri dishes and from the air to rate the capacity of these hand-drying devices to scatter contaminants into the surrounding environment.
Blowing Viruses Throughout the Room
The scientists discovered the jet air hand dryers could disperse viruses up to nine feet from the device. By contrast, the more commonly used and less powerful warm air dryer spewed the MS2 three feet from the machine. Paper towels were only able to disperse the virus a mere 10 inches.

Based on research originally published in the Journal of Hospital Infection, the graphic above demonstrates how the various hand-drying methods alter the spread of viruses, described by Westminster researchers. These findings will be of interest to microbiologists, pathologists and medical laboratory scientists involved in infection-control programs at their hospitals and labs. (Graphic copyright: Food Safety Consortium, Ltd.)
The type of hand dryer used for the study was the Dyson Airblade. The researchers learned that the high-powered Airblade spread 60 times more germs into the air than the lower-powered warm air dryers and scattered 1,300 more viruses than the paper towels.
Dyson criticized the study, noting that the scientists had an unusually high amount of the virus on their hands. The company also stated that while paper towels may not dispense viruses into the air, they can be polluted with germs and spread them to other people. In addition, Dyson claims on its website that “up to 88% of unused paper towels tested in the US contain bacteria, which can transfer to your hands.”
Dyson has also alleged that such studies are funded by the paper towel industry to discredit the effectiveness of their products.
Thorough Hand Washing a Critical Step
In addition to having a large amount of the virus on their hands, it is worth noting that the researchers did not attempt to wash the MS2 from their hands before using the assorted drying techniques. People typically have washed their hands with soap and water before operating any type of hand dryer or wiping their hands with paper towels. Although it is debatable which hand-drying method is the most hygienic, obviously the best practice is to thoroughly wash hands and dry them with whatever hand-dryer is available.
Hand hygiene is widely known to be a crucial element in minimizing the transmission of pathogenic micro-organisms that can cause infections. According to the Westminster study, “it has been estimated that cross-infection contributes to 40% of cases of healthcare-associated infections and hand hygiene compliance represents an essential step in minimizing such infections.”
Choosing Best Hand Dryer for Medical Environments, Clinical Laboratories
The researchers noted that, “the choice of hand-drying device should be considered carefully in areas where infection prevention concerns are paramount, such as healthcare settings and the food industry.”
In the past, microbiologists have performed studies where they have swabbed the hands of medical staff, equipment, and surfaces to demonstrate the presence of infectious agents. One study even examined doctors’ neckties and found the existence of bacteria that can cause infections, such as:
· Klebsiella pneumoniae;
· Pseudomonas aeruginosa;
· Staphylococcus aureus; and
· Aspergillus fungus.
In 2013, Weill Cornell Medical College launched PathoMap to study genetic material in the New York City Subway System. Their objective was to establish a molecular view of the city to positively impact public health.
Weill researchers discovered genetic material from more than 15,000 species among 1,400 samples collected from 468 subway stations. The material was mostly harmless or unidentified.
PathoMap recently implemented MetaSUB, which stands for “Metagenomics and Metadesign of Subways and Urban Biomes,” to perform similar studies of mass-transit systems in 39 cities on six continents. The goal is to help city planners, public health officials, and designers create healthier environments.
Whether “virus hand grenades” are fact or myth, targeted research such as the studies above highlight the critical need for clinical laboratories and other medical practices to understand how the devices used in hand washing and hand drying contribute to improved hygiene and lower infection rates that help protect patients as well as physicians, nurses, medical laboratory scientists, and other healthcare workers.
—JP Schlingman
Related Information:
Study: ‘Jet’ Hand Dryers Act Like Virus Hand Grenades
Hand Dryer vs. Paper Towel: Which Is Cleaner?
Comparison of Different Hand-Drying Methods: The Potential for Airborne Microbe Dispersal and Contamination
Dyson Hand Dryers Spread More Germs than Paper Towels, Study Says
Do Jet Hand Dryers Really Spread More Germs than Paper Towels?
Evaluation of the Potential for Virus Dispersal During Hand Drying: A Comparison of Three Methods
Study Finds Doctors’ Neckties Carry Pathogens
There are 15,000 Lifeforms Riding the NYC Subway, Including Meningitis
Up To 88% of Unused Paper Towels Tested in the US Contain Bacteria, Which Can Transfer to Your Hands
Other Hand Dryers Can Blow Viruses and Bacteria onto Your Hands, Some of It from Feces
Microbiologists at Weill Cornell Use Next-Generation Gene Sequencing to Map the Microbiome of New York City Subways







