Sep 10, 2018 | Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
Clinical laboratory test claims make up a substantial proportion of all claims filed each year. Thus, any effort to streamline or reform claims adjudication and administration in the US will alter how labs and pathologists conduct business
Clinical laboratory managers and anatomic pathologists know how costly and complex the US healthcare system can be. However, expenses associated with care and treatment are only part of the total picture. Resources devoted to paperwork and administrative costs apparently increase overall expenditures associated with healthcare to a much higher degree than is generally known.
That’s according to several studies The New York Times reported on in July.
US Administrative Costs Higher than All Other Nations
One study conducted by The New England Journal of Medicine (NEJM) in 2003 estimated administrative costs account for approximately 30% of all healthcare expenditures in the US. The researchers examined data from 1999 to reach those conclusions. In today’s economy, those numbers are higher. On average, $5,700 of every $19,000 that US workers and their employers pay for family coverage each year goes towards administrative costs.
A 2014 study published by Health Affairs compared administrative costs for US hospital expenditures to those of seven other countries: Canada, England, France, Germany, the Netherlands, Scotland, and Wales. This study evaluated data from 2010/2011 and found that hospital administrative costs in the US far exceed rates in other nations. According to the study, administrative costs accounted for:
- 25.3% of total hospital expenditures in the US;
- 19.8% in the Netherlands;
- 15.5% in England; and,
- 12% in Canada and Scotland.
According to the Health Affairs study, more than $150 billion could have been saved in 2011 by reducing per capita spending for administrative costs to the levels observed in Canada and Scotland.

“The extraordinary costs we see are not because of administrative slack or because healthcare leaders don’t try to economize,” Kevin Schulman, MD, Professor, Department of Medicine, Duke University, and co-author of the Health Affairs study told The New York Times. “The high administrative costs are functions of the system’s complexity.” (Photo copyright: Duke University.)
Complexity of Payer System Partly to Blame
One reason for the costliness in the US healthcare system is the myriad of payers that healthcare organizations have to grapple with to receive payment. Private health insurers and public health programs like Medicare and Medicaid, each have their own procedures, regulations, and forms that need to be submitted to receive payments. This translates to more employee time devoted to billing.
Another factor driving costs is the staff time devoted to the collection of debts. A 2017 Health Affairs study examined medical claims data from 88,000 healthcare providers contracted with Athenahealth to determine the percentage of bills paid within one year from the initial service.
The study found that 93.8% of patient bills under $35 were paid within a year. However, that percentage decreased as the patient obligation increased:
- 90.5% of patients paid bills between $35 and $75 within one year;
- 83.7% paid bills between $75 and $200 in the same time period; however,
- When bills increase to $200 or more, just 66.7% were paid within a year’s time.
Providers wrote off approximately 16% as abandoned or bad debts, with an additional 17% going to collection agencies.
Another study, published in Health Affairs in 2009, surveyed 895 physicians about the time they spent dealing with administrative tasks. On average, physicians reported spending 43 minutes per workday interacting with health plans. This number is the equivalent of three hours/week and almost three weeks/year. Those numbers have reportedly increased since then.
EHRs Do Not Reduce Administrative Costs, Contrary to Belief
Efforts have been made to reduce administrative costs in the US healthcare industry. One such measure involved increased use of certified electronic health record (EHR) systems, which the federal government spent billions of dollars promoting and incentivizing providers to adopt on the claim that EHRs would reduce healthcare costs, in part by removing most of the paperwork.
However, a 2018 study published in the Journal of the American Medical Association (JAMA) reported the adoption of EHRs did not reduce administration costs. Researchers at Duke University and Harvard Business School utilized a cutting-edge accounting method to determine the administrative costs within a large academic healthcare system that was using a certified EHR.
Their study determined the administrative costs for processing a single medical bill ranged from $20 for a doctor visit to $215 for an inpatient surgical procedure. These costs accounted for 3%-25% of total professional revenue for the provided services.
“We need to understand better how complexity is driving these enormous costs within the system, costs that do not add value to patients, employers, or providers,” noted Barak Richman, JD, PhD, Duke University School of Law and Margolis Center for Health Policy, one of the study’s authors.
Clinical Lab Test Claims a Major Portion of Administrative Costs
Nevertheless, administrative costs are a necessary part of doing business and not always as negative as perceived. An article published by Health Affairs in 1992 divided administrative costs in the healthcare industry into four categories:
- Transaction-related: claims processing, billing, admissions, and tracking employee hiring/terminations;
- Benefits Management: quality assurance, plan design, statistical and internal analyses, and management information systems;
- Selling and Marketing: strategic planning, underwriting, and advertising; and,
- Regulatory and Compliance: waste management, licensing requirements, and discharge planning.
“We hope that this work is the first step toward informing policy solutions that could reduce these non-value-added costs largely hidden within the healthcare system,” Schulman stated in a Duke University news release.
The issue of costly paperwork and administrative expenditures is significant for the clinical laboratory profession as lab test claims make up a substantial portion of all medical claims filed annually. Efforts to streamline or reform claims adjudication and administration will have an impact on the way clinical labs and anatomic pathology groups conduct business in the future.
—JP Schlingman
Related Information:
Hidden from View: The Astonishingly High Administrative Costs of U.S. Health Care
NEJM: Costs of Health Care Administration in the United States and Canada
Heath Affairs: A Comparison of Hospital Administrative Costs in Eight Nations: US Costs Exceed All Others by Far
Heath Affairs: Inside the Black Box of Administrative Costs
Heath Affairs: As Patients Take on More Costs, Will Providers Shoulder the Burden?
Heath Affairs: What Does It Cost Physician Practices to Interact with Health Insurance Plans?
Electronic Health Records Don’t Reduce Administrative Costs
Simplifying Administration of Health Insurance
Sep 5, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory Pathology, Laboratory Testing
While approaches differ between the three companies, heavy investment in EMR/EHR and other HIT solutions could signal significant changes ahead for a market currently dominated by only a few major developers
If healthcare big data is truly a disruptive force in healthcare’s transformation, then a big battle looms for control of that data. Some experts say that the companies now dominating the electronic health record (EHR) market will soon face tough competition from the world’s biggest tech companies.
Until recently, most clinical laboratories, anatomic pathology groups, hospitals, and other healthcare providers have depended on EHR systems from just a handful of health information technology (HIT) developers. But tech giants Google, Apple, and Microsoft have been filing hundreds of HIT related patents since 2013 and appear poised to compete on a large scale for a chunk of the EMR/EHR/HIT market, according to coverage in EHR Intelligence of Kalorama Information’s “EMR 2018: The Market for Electronic Medical Records” report.
How this will impact medical laboratories and pathology practices remains to be seen. Labs are sure to be influenced by coming events, since clinical laboratory test data represents the largest proportion of an individual patient’s permanent medical record. It’s important to note, though, that while most EHR/HIT developers have been motivated by federal incentives, Google (NASDAQ:GOOG), Apple (NASDAQ:AAPL), and Microsoft (NASDAQ:MSFT) are motivated by consumer demand, which increasingly dictates the direction of health technology development.
Thus, they may be better positioned to compete moving forward, as patients, physicians, and hospitals turn to precision medicine and value-based care for improved outcomes and increased revenues.

“The EMR efforts have moved hospitals from paper to digital records,” Bruce Carlson (above), Publisher of Kalorama Information, told HIT Infrastructure. “The next step is for tech giants to glean the data and improve upon that infrastructure. We’ll be talking about EHR in different ways in the next ten years.” (Photo copyright: Twitter.)
EMR/EHR Market Poised for Disruption
According EHR Intelligence, as of 2017, 97% of all US non-federal acute care hospitals and 84% of US hospitals had adopted an EHR system. Of these hospitals, more than half (50.5%) use products from just two developers—Cerner or Epic. That’s according to Health Data Management’s coverage of the KLAS report “US Hospital EMR Market Share 2017.”
However, recent interest in HIT and EHR systems by major Silicon Valley tech companies could lead to potential disruptions in the current state of the market. According to The New York Times, in the first 11 months of 2017, 10 of the largest US technology companies were involved in healthcare equity deals worth $2.7-billion. This marks a drastic increase over the 2012 figure of $277-million.
Though each company is approaching the market differently, Google, Microsoft, and Apple are all working on projects that could influence how both consumers and healthcare professionals interact with and utilize medical record data.
Of the three, Apple is the most consumer-centric with their Apple Health personal health record (PHR) integration into Apple iOS for iPhones and iPads. Microsoft, however, is working on developing analytics tools and storage solutions aimed at healthcare providers in general. And Google, through its parent company Alphabet, is focusing on data processing and storage.
Amazon also is working on its own HIT project which it calls 1492. While details are scant, HIT Infrastructure reports that the project is focused on interoperability among disparate EHR systems to improve sharing of protected health information (PHI) between providers, patients, and other healthcare providers, such as clinical labs and pathology groups. HIT Infrastructure also reported on rumors of Amazon branching into telemedicine using their Amazon Echo and Alexa platforms.
Security Concerns and Opportunities for Clinical Laboratories
According to Computerworld’s coverage of IDC research, by 2020, 25% of patients are expected to be taking part in ‘bring your own data” healthcare scenarios. Tech-savvy medical laboratories could find opportunities to interact directly with patients and encourage follow-through on test orders or follow-up on routine testing.
However, shifting protected health information to devices carried by consumers is not without risks.
“How do I know the data won’t make its way to some cloud somewhere to be shared, sold, etc.” Jack Gold, Principal Analyst with J. Gold Associates, told Computerworld. “And if I rely on an app to tell me what to do—say, take my meds—and it somehow gets hacked, can it make me sick, or worse?”
These are important questions and developments, which Dark Daily has covered in other recent e-briefings. (See, “Apple Updates Its Mobile Health Apps, While Microsoft Shifts Its Focus to Artificial Intelligence. Both Will Transform Healthcare, But Which Will Impact Clinical Laboratories the Most?” July 25, 2018.)
Nevertheless, with tech giants already developing products for the consumer market and healthcare provider industry, it’s a given consumers will soon gain greater access to their own healthcare information. Whether patients will ultimately embrace it, how they will use it, and how developers will interact with the data, is still undefined. But it’s coming and clinical laboratories should be prepared.
—Jon Stone
Related Information:
Apple to Launch Health Records App with HL7’s FHIR Specifications at 12 Hospitals
How Google, Microsoft, Apple Are Impacting EHR Use in Healthcare
Microsoft, Apple, Google Secure HIT Infrastructure Patents
How Big Tech Is Going after Your Health Care
Amazon Secret Healthcare IT Tech Team Focuses on EHRs, Alexa
Apple’s Health Record API Released to Third-Party Developers; Is It Safe?
Apple, Cerner and Microsoft Are Interested in Buying AthenaHealth: Here’s Why This CEO Says They Won’t
Apple Says iOS Health Records Has over 75 Backers, Uses Open Standards
Report: Health Systems Share Apple Health Records Feedback
Apple Is Officially in the EHR Business. Now What?
Why Apple’s Move on Medical Records Marks a Tectonic Shift
Slideshow Where the Top 8 EMRs Are Deployed
Apple Updates Its Mobile Health Apps, While Microsoft Shifts Its Focus to Artificial Intelligence. Both Will Transform Healthcare, but Which Will Impact Clinical Laboratories the Most?
Apple’s Update of Its Mobile Health App Consolidates Data from Multiple EHRs and Makes It Easier to Push Clinical Laboratory Data to Patients
Apr 6, 2018 | Compliance, Legal, and Malpractice, Digital Pathology, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Management & Operations, News From Dark Daily
Softened FDA regulation of both clinical-decision-support and patient-decision-support software applications could present opportunities for clinical laboratory developers of such tools
Late 2017, the Food and Drug Administration (FDA) released guidelines on how the agency intends to regulate—or not regulate—digital health, clinical-decision-support (CDS), and patient-decision-support (PDS) software applications. The increased/decreased oversight of the development of these physicians’ tools could have important implications for anatomic pathology groups and clinical laboratories.
Physician decision-support software utilizes medical laboratory test data as a significant part of a full dataset used to guide caregivers. Thus, if the FDA makes it easier for developers to get regulatory clearance for these types of products, that could positively impact medical labs’ ability to service their client physicians.
Additionally, clinical pathologists have unique training in diagnosing diseases and understanding the capabilities and limitations of medical laboratory tests in supporting how physicians diagnose disease and make treatment decisions. Thus, actions by the FDA to make it easier for developers of software algorithms that can incorporate clinical laboratory data and anatomic pathology images with the goal of improving diagnoses, decisions to treat, and monitoring of patients have the potential to bring great benefit to the nation’s medical laboratories.
FDA Clarifies Role in Regulating CDS/PDS Applications
The new guidelines clarified items specified in the 21st Century Cures Act, which was enacted by Congress in December of 2016. This Act authorized $6.3 billion in funding for the discovery, development, and delivery of advanced, state-of-the art medical cures.
“Today, we’re announcing three new guidances—two draft and one final—that address, in part, important provisions of the 21st Century Cures Act, that offer additional clarity about where the FDA sees its role in digital health, and importantly, where we don’t see a need for FDA involvement,” FDA commissioner Scott Gottlieb, MD, Commissioner of Food and Drugs, noted in a statement. “We’ve taken the instructions Congress gave us under the Cures Act and [we] are building on these provisions to make sure that we’re adopting the full spirit of the goals we were entrusted with by Congress.”
Helping Doctors’ Decision-Making
The first guideline concerns clinical decision support systems that are designed to help doctors make data-driven decisions about patient care. The new guidelines make it easier for software developers to get regulatory clearance, which, the FDA hopes, will spark innovation and makes regulation more efficient.
“CDS has many uses, including helping providers, and ultimately patients, identify the most appropriate treatment plan for their disease or condition,” Gottlieb said in the FDA’s statement. “For example, such software can include programs that compare patient-specific signs, symptoms, or results with available clinical guidelines to recommend diagnostic tests, investigations or therapy.
“This type of technology has the potential to enable providers and patients to fully leverage digital tools to improve decision making,” Gottlieb continued. “We want to encourage developers to create, adapt, and expand the functionalities of their software to aid providers in diagnosing and treating old and new medical maladies.”
Identifying Digital Health Applications That Receive/Don’t Receive FDA Oversight
The second guideline discusses and delineates which digital health applications are considered low risk and, thus, will not fall under FDA regulations.
Products that are not intended to be used for the diagnosis, cure, mitigation, prevention, or treatment of a condition will not be regulated by the FDA. These technologies are not considered medical devices and may include gadgets such as weight management and mindfulness tools. They can provide value to consumers and the healthcare industry while posing a low risk to patients.
“Similarly, the CDS draft guidance also proposes to not enforce regulatory requirements for lower-risk decision support software that’s intended to be used by patients or caregivers—known as patient-decision-support software (PDS)—when such software allows a patient or a caregiver to independently review the basis of the treatment recommendation,” Gottlieb noted in the statement.

Scott Gottlieb, MD (above), FDA Commissioner of Food and Drugs, noted in a statement, “We believe our proposals for regulating CDS and PDS not only fulfill the provisions of the Cures Act, but also strike the right balance between ensuring patient safety and promoting innovation. Clinical laboratories may find opportunities to work with CDS/PDS developers and support their client physicians. (Photo copyright: FDA.)
However, products that are intended to be used for the diagnosis, cure, mitigation, prevention, or treatment of a condition are considered medical devices and will fall under FDA regulations.
“The FDA will continue to enforce oversight of software programs that are intended to process or analyze medical images, signals from in vitro diagnostic devices, or patterns acquired from a processor like an electrocardiogram that use analytical functionalities to make treatment recommendations, as these remain medical devices under the Cures Act,” noted Gottlieb.
Items such as mobile apps that are utilized to maintain and encourage a healthy lifestyle are not deemed to be medical devices and will fall outside FDA regulations. The guidelines also defined that Office of the National Coordinator for Health Information Technology (ONC)-certified electronic health record (EHR) systems are not medical devices and, thus, will not be regulated by the FDA.
Software-as-a-Medical Device Gets FDA Oversight
The third guidance document deals with the assessment of the safety, performance, and effectiveness of Software as a Medical Device (SaMD).
“This final guidance provides globally recognized principles for analyzing and assessing SaMD, based on the overall risk of the product. The agency’s adoption of these principles provides us with an initial framework when further developing our own specific regulatory approaches and expectations for regulatory oversight and is another important piece in our overarching policy framework for digital health,” Gottlieb noted in the statement.
SaMD is defined by the International Medical Device Regulators Forum (IMDRF) as “software intended to be used for one or more medical purposes that perform these purposes without being part of a hardware medical device.”
Gottlieb noted that the three important guidance documents being issued would continue to expand the FDA’s efforts to encourage innovation in the ever-changing field of digital health. “Our aim is to provide more clarity on, and innovative changes to, our risk-based approach to digital health products, so that innovators know where they stand relative to the FDA’s regulatory framework. Our interpretation of the Cures Act is creating a bright line to define those areas where we do not require premarket review,” he concluded.
What remains to be seen is how the new FDA regulations will impact clinical laboratories and anatomic pathology groups. With the expanding interest in artificial intelligence (AI) and self-learning software systems, healthcare futurists are predicting a rosy future for informatics products that incorporate these technologies. Hopefully, with these new guidelines in place, innovative clinical laboratories will have the opportunity to develop new digital products for their clients.
—JP Schlingman
Related Information:
FDA Softens Stance on Clinical-decision Support Software
Clinical and Patient Decision Support Software
FDA Issues New Guidance for Clinical and Patient Decision Support Software
Statement from FDA Commissioner Scott Gottlieb, M.D., on Advancing New Digital Health Policies to Encourage Innovation, Bring Efficiency and Modernization to Regulation
FDA Issues Three Guidances, Including Long-awaited CDS Guidelines
The Feds Just Cleared a Major Roadblock for Digital Health
FDA Unveils Clinical Decision Support, Medical Device Guidance
Mar 23, 2018 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory Pathology, Management & Operations
Consumer demand for health trackers combined with other smartwatch capabilities is driving a trend away from simple health trackers and toward more complex devices, such as the Apple Watch, for their more powerful capabilities
It is still an open question as to whether clinical laboratories will experience an onrush of patient test data streaming at them from healthcare consumer portals and mobile devices. The popularity of wearable fitness/medical technology has been widely touted in the media. Predictions have been that these devices—when coupled with smartphone and tablet applications (apps)—would generate substantial volumes of digital patient data that would be useful for medical laboratories to capture and add to the clinical lab test data of the patients they serve.
But will these predictions of a flood of data from wearable devices become reality? Is this a trend about which medical laboratories should be concerned? Recent statistics provide some insight into these questions. For example, the sales numbers for wearable devices are significant.
Smartwatches Gaining Ground in Wearable Fitness Market
In 2016, 102.4 million wearable devices were sold, which was a 25% increase over the previous year, according to Smart Insights, a publisher for marketers. Now, several sports apparel companies, such as Adidas and Under Armour, are either launching smartwatches with health/fitness-related software and activity trackers, or eliminating their digital fitness business units altogether.
And according to MobiHealthNews, “[today’s] landscape looks awfully different.
“I think the industry is still struggling to find real, meaningful points of reference with consumers,” Dan Ledger, Principal and Founder, Path Collaborative, a Massachusetts consulting firm, told MobiHealthNews. “You hear anecdotes of people who had Fitbit (NYSE:FIT) and lost weight. But it hasn’t really been a success as a market product like a smartphone—like a lot of these companies were expecting when they were reading the tea leaves four or five years ago.”
For example, Adidas reassigned employees working in the fitness watch and sensor-enabled footwear departments to other areas, according to the Portland Business Journal. “We are integrating digital across all areas of our business and will continue to grow our digital expertise but in a more integrated way,” an Adidas spokesperson told Just-Style.
And, Nike announced its intention late last year to abandon the wearables market altogether. “It wasn’t authentic to who we were,” Jordan Rice, Senior Director of Nike NXT Smart Systems Engineering, told MobiHealthNews.
Meanwhile, Under Armour announced in 2017 that it planned to eliminate the UA HealthBox, a wearable device that offered a connected activity tracker, heart rate monitor, and smart scale tools, according to MHealth Spot. Instead, the publication reported, Under Armour was partnering with Samsung on fitness apps:
- MyFitnessPal;
- MapMyFitness;
- Endomondo; and,
- UA Record.
More Consumers Strapping on Smartwatches
Fitbit recently released the Fitbit Ionic Watch. According to Fitbit’s website, features include:
- Personal coaching;
- Heart rate monitor;
- All-day activity tracking;
- Sleep stages monitoring; and more.

The smartwatch may be the new “smart” way to go, compared to simple activity trackers. Smartwatch manufactures are partnering with biometric monitoring app developers (such as Apple Watch and IBM Watson Health, shown above) to service consumers who need to monitor, capture, and distribute their critical health data. (Photo copyright: Alexey Boldin/Shutterstock.)
Consumer Reports, citing NPD Group market data, noted smartwatches are increasingly becoming the device-of-choice for consumers who gather fitness data. Besides tracking heart rate, some smartwatch apps also release notifications about accomplishment of goals, enable access to e-mail, and more.
Consumer Reports noted:
- Smartwatches were used by 17% of US adults in the first quarter of 2015, and the remaining 83% in the demographic used activity trackers;
- Smartwatch use jumped to 38% by the fourth quarter of 2017; and,
- Smartwatches will rise to 48% of new market purchases by the fourth quarter this year.
Hardware is Hard
Fitness wearable devices have long been touted by the media for their potential to stream critical health data directly to physicians, to patients’ electronic health records, and to medical laboratories. Dark Daily foresaw in 2016 that, when paired with a smartphone or table computer, the momentum of the fitness wearables trend was substantial. For this reason, clinical laboratory managers and pathologists would want to stay current with these developments. However, today it appears companies offering wearable monitoring devices could be finding it more difficult than anticipated to capture the attention of consumers and leverage what the devices do.
In the end, sports apparel companies are not leaving the digital fitness space entirely, but simply adjusting to new consumer demands. Clinical laboratory leaders will want to keep watch on these developments as the trend evolves. The outcome could alter how patient data enters the pathology workflow.
—Donna Marie Pocius
Related Information:
Digital Marketing Strategy Wearables Statistics 2017
Sports Apparel Brands are All Walking Away from Fitness Wearables
Under Armour Kills the HealthBox Suite of Connected Devices
Adidas to Cut Digital Sports Division
Fitness Tracker or Smartwatch: Which is Best for You?
Improvements to Fitness Wearables Help Stream Data from Consumers Homes to EHRs and Clinical Pathology Laboratories
Mar 21, 2018 | Digital Pathology, Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Sales and Marketing, Laboratory Testing, News From Dark Daily
January’s press release confirmed the tech company is working to integrate critical medical data into its mobile devices, while further promoting interoperability and patient access
While interoperability has improved since the earliest electronic health record (EHR) systems, today’s active patients often need to sort through multiple healthcare portals—including those of clinical laboratories and anatomic pathology groups—to get a comprehensive view of their medical history. Not only can this be time consuming, but also inconvenient if the patient lacks access to a computer.
Thus, it’s no surprise that in a January 24 press release, mobile technology giant Apple announced plans to enter the development ring and create an improved EHR for its mobile device users by updating its existing “Health” mobile application (app). The iOS 11.3 update, among other things, is designed to enable Apple iPhone owners to receive critical medical data, such as medical laboratory test results, directly on their devices.
“Our goal is to help consumers live a better day. We’ve worked closely with the health community to create an experience everyone has wanted for years—to view medical records easily and securely right on your iPhone,” said Apple COO Jeff Williams in the press release.

Jeff Williams (above), COO at Apple, notes that, “By empowering customers to see their overall health, we hope to help consumers better understand their health and help them lead healthier lives.” (Photo copyright: Apple.)
The new features are already available to developers in the latest iOS 11.3 beta 3 release. However, release to the public is expected soon with the issuance of the iOS 11.3 final release. This means that patients will not need to download extra apps—or remember to use them—to take advantage of the feature.
New Way to Improve Patients’ Access to Health Data or Just Another Data Silo?
The Apple Health Records platform adheres to Fast Healthcare Interoperability Resources (FHIR) protocols for transmission of data. Providers send information to Apple which then aggregates the information, transmits it to patients’ iPhones and notifies them of the updates.
All information stored on the device is encrypted in storage and protected from unauthorized access by the user’s password.
Through the new Health Records interface, users view this aggregated data as a timeline, conduct searches, and share information with other parties as they deem appropriate.
Current medical information listed in the press release includes:
- Allergies;
- Conditions;
- Immunizations;
- Clinical laboratory results;
- Medications;
- Procedures; and,
- Vitals.
Currently, the platform integrates data from three major EHR developers:
- Epic;
- Cerner; and,
- AthenaHealth
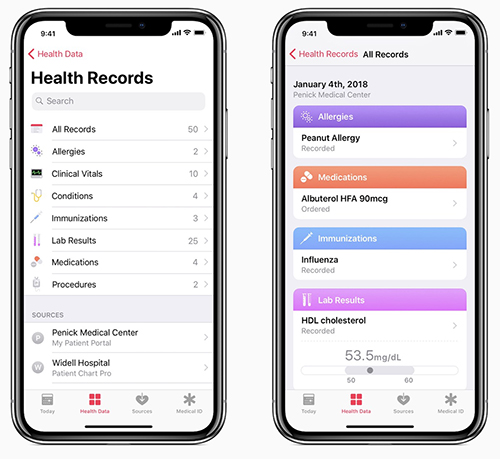
Apple’s update to the Health app makes it easier for people to access and control of all of their health records and data. This included medical laboratory tests. (Image and caption copyright: Apple.)
Apple is also working with 12 health institutions across the US in the first phase of the project, including:
In the Apple press release, Stephanie Reel, CIO at John Hopkins Medicine in Baltimore, stated, “Streamlining information sharing between patients and their caregivers can go a long way towards making the patient experience a positive one. This is why we are excited about working with Apple to make accessing secure medical records from an iPhone as simple for a patient as checking email.”
Previous Attempts at Mobile Health Record Devices Got Mixed Results
This isn’t the first time a major technology company has attempted to enter the mobile health market. Google Health was shuttered in 2011 citing low adoption. Wearable fitness trackers, such as Fitbit (NYSE:FIT) enjoyed a bubble, but are now seeing mixed success in terms of long-term adoption and use, according to The Motley Fool. More to the point, they’ve never quite become the holy grail of monitoring and data collection that some experts predicted, Huffington Post reported.
However, Apple’s investments and interest in healthcare-related technologies has led to wide speculation that they would enter the health market this year. (See Dark Daily “Apple May Be Developing Mobile Device Technology to Monitor User’s Health and Transmit Data in Real Time.”)
Larry Dignan, Editor-in-Chief at ZDNet, builds a compelling case for why this could be the attempt that succeeds in providing a consolidated platform for clinical laboratories, physicians, and other care providers to push data directly to patients and—with the patient’s permission—to each other, regardless of the platforms healthcare facilities use to store and transmit data.
He notes that much of Apple’s newest features build on foundations laid by the healthcare industry to create scalable, functional EHR systems. By working with existing protocols, Apple’s Health Records platform is already positioned for compatibility with many healthcare providers.
Furthermore, Apple is already known for partnering at the enterprise level with major businesses and industries, while also holding the trust of millions of Americans who store their personal information on Apple devices.
Is Apple the Future of EHRs?
Despite this, until the platform—and adoption by the public—is proven a success, it will be yet another walled garden of medical information. Even then, Apple is only one segment of the global mobile market.
Unless Apple provides access to other platforms (such as Android), those patients—and the medical communities serving them—are left consolidating information on their own through a sprawl of various portals. This also means that medical laboratories, pathology groups, and other service providers must continue to invest time and funding into communicating data in ways compatible with a plethora of internal and external systems and software.
Still, the platform offers an intriguing glimpse at the future of medical records and heralds a shift toward empowering patients with easy, comprehensive access to their own data, which would be a boon to the medical laboratory industry.
—Jon Stone
Related Information:
Apple Previews iOS 11.3
Apple Announces Effortless Solution Bringing Health Records to iPhone
With Medical Records Tools, Apple Wades Deeper into Digital Health
Apple Confirms “Health Records” Solution with Aim to Bring Medical Records to iPhone
Apple Will Let You Keep Your Medical Records on Your iPhone
Apple Unveils mHealth Integration with EMR Data through Health App
Apple, Inc. Wants to Solve the Problem of Electronic Health Records
Viewpoint: How Realistic Is Apple’s Attempt at the EHR Industry? Very—6 Reasons Why
Apple Can Win Electronic Medical Record Game with Health Records in iOS 11.3: Here’s 7 Reasons Why
Apple Is Officially in the EHR Business. Now What?
Apple to Launch Health Records App with HL7’s FHIR Specifications at 12 Hospitals
Could Amazon or Apple Actually Make a Dent in the EHR Market?
Apple May Be Developing Mobile Device Technology to Monitor User’s Health and Transmit Data in Real Time









