Apr 13, 2018 | Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Management & Operations, News From Dark Daily
PwC’s list of 12 factors that will shape the healthcare landscape in 2018 calls attention to many new innovations Dark Daily has reported on that will impact how medical laboratories perform their tests
PwC’s Health Research Institute (HRI) issued its annual report, detailing the 12 factors expected to impact the healthcare industry the most in 2018. Dark Daily culled items from the list that will most likely impact clinical laboratories and anatomic pathology groups. They include:
How clinical laboratory leaders respond to these items could, in part, be determined by new technologies.
AI Is Everywhere, Including in the Medical Laboratory
Artificial intelligence is becoming highly popular in the healthcare industry. According to an article in Healthcare IT News, business executives who were polled want to “automate tasks such as routine paperwork (82%), scheduling (79%), timesheet entry (78%), and accounting (69%) with AI tools.” However, only about 20% of the executives surveyed have the technology in place to use AI effectively. The majority—about 75%—plan to invest in AI over the next three years—whether they are ready or not.
One such example of how AI could impact clinical laboratories was demonstrated by a recent advancement in microscope imaging. Researchers at the University of Waterloo (UW) developed a new spectral light fusion microscope that captures images in full color and is far less expensive than microscopes currently on the market.
“In medicine, we know that pathology is the gold standard in helping to analyze and diagnose patients, but that standard is difficult to come by in areas that can’t afford it,” Alexander Wong, PhD, one of the UW researchers, told CLP.
“The newly developed microscope has no lens and uses artificial intelligence and mathematical models of light to develop 3D images at a large scale. To get the same effect using current technologies—using a machine that costs several hundred thousand dollars—a technician is required to ‘stitch together’ multiple images from traditional microscopes,” CLP noted.
Healthcare Intermediaries Could Become Involved with Clinical Laboratory Data
Pricing is one of the biggest concerns for patients and government entities. This is a particular concern for the pharmaceutical sector. PwC’s report notes that “stock values for five of the largest intermediaries in the pharmacy supply chain have slumped in the last two years as demands for lower costs and better outcomes have intensified.”
Thus, according to PwC, pressure may come to bear on intermediaries such as Pharmacy Benefit Managers (PBMs) and wholesalers, to “prove value and success in creating efficiencies or risk losing their place in the supply chain.”
Similar pressures to lower costs and improve efficiency are at work in the clinical laboratory industry as well. Dark Daily reported on one such cost-cutting measure that involves shifting healthcare payments toward digital assets using blockchains. The technology digitally links trusted payers and providers with patient data, including medical laboratory test results. (See, “Blockchain Technology Could Impact How Clinical Laboratories and Pathology Groups Exchange Lab Test Data,” September 29, 2017.)

PwC’s latest report predicts 12 forces that will continue to impact healthcare, including clinical laboratories and anatomic pathology groups, in 2018. Click on the image of the cover above to access an online version of the report. (Photo copyright: PwC/Issuu.)
The Opioid Crisis Remains at the Forefront
Healthcare will continue to feel the impact of the opioid crisis, according to the PwC report. Medical laboratories will continue to be involved in the diagnosis and treatment of opioid addition, which has garnered the full attention of the federal government and has become a multi-million-dollar industry.
Security Remains a Concern
Cybersecurity will continue to impact every facet of healthcare in 2018. Healthcare IT News reported, “While 95% of provider executives believe their organization is protected against cybersecurity attacks, only 36% have access management policies and just 34% have a cybersecurity audit process.”
Patients are aware of the risks and are often skeptical of health information technology (HIT), Dark Daily reported in June of last year. Clinical laboratories must work together with providers and healthcare organizations to audit their security measures. Recognizing the importance of the topic, the National Independent Laboratory Association (NILA) has named cybersecurity for laboratory information systems (LIS) a focus area.
Patient Experience a Priority
Although there have been significant improvements in the area of administrative tasks, there is still an enormous demand for a better patient experience, including in clinical laboratories. Healthcare providers want patients to make changes for the better that ultimately improve outcomes and the patient experience is one path toward that goal.
“Provider reimbursements will be based in part on patient engagement efforts such as promoting self-management and coaching patients between visits,” PwC noted in its report, a fact that Dark Daily has continually reported on for years. (See, “Pathologists and Clinical Lab Executives Take Note: Medicare Has New Goals and Deadlines for Transitioning from Fee-For-Service Healthcare Models to Value-Based Reimbursement,” April 1, 2015.)
Demands for Price Transparency Increase
As they follow healthcare reform guidelines to increase quality while lowering costs, state governments will continue to ramp up pressure on healthcare providers and third parties in the area of pricing. Rather than simply requiring organizations to report on pricing, states are moving towards legislating price controls, as Dark Daily reported in February.
Social Factors Affect Healthcare Access
The transition to value-based care makes the fact that patients’ socioeconomic statuses matter when it comes to their health. “The most important part of getting good results is not the knowledge of the doctors, not the treatment, not the drug. It’s the logistics, the social support, the ability to arrange babysitting,” David Berg, MD, co-founder of Redirect Health told PwC.
One such transition that is helping patients gain access to healthcare involves microhospitals and their adoption of telemedicine technologies, which Dark Daily reported on in March.
“Right now, they seem to be popping up in large urban and suburban metro areas,” Priya Bathija, Vice President, Value Initiative American Hospital Association, told NPR. “We really think they have the potential to help in vulnerable communities that have a lack of access.”
Data Collection Challenges Pharma
The 21st Century Cures Act, along with the potential exploitation of Big Data, will make it possible for organizations to gain faster, less expensive approvals from the US Food and Drug Administration (FDA). As Dark Daily noted in April, the FDA “released guidelines on how the agency intends to regulate—or not regulate—digital health, clinical-decision-support (CDS), and patient-decision-support (PDS) software applications.
“Physician decision-support software utilizes medical laboratory test data as a significant part of a full dataset used to guide caregivers,” Dark Daily noted. “Thus, if the FDA makes it easier for developers to get regulatory clearance for these types of products, that could positively impact medical labs’ ability to service their client physicians.”
Healthcare Delivery During and Following Natural Disasters
PwC predicts the long-term physical results, financial limitations, and supply chain disruptions following natural disasters will continue to affect healthcare in 2018. The devastation can prevent many people from receiving adequate, timely healthcare.
However, new laboratory-on-a-chip (LOC) and other “lab-on-a-…” testing technologies, coupled with medical drone deliver services, can bring much need healthcare to remote, unreachable areas that lack electricity and other services. (See Dark Daily, “Lab-on-a-Fiber Technology Continues to Highlight Nano-Scale Clinical Laboratory Diagnostic Testing in Point-of-Care Environments,” April 2, 2018, and, “Johns Hopkins’ Test Drone Travels 161 Miles to Set Record for Delivery Distance of Clinical Laboratory Specimens,” November 15, 2017.)
PwC’s report is an important reminder of from where the clinical laboratory/anatomic pathology industry has come, and to where it is headed. Sharp industry leaders will pay attention to the predictions contained therein.
—Dava Stewart
Related Information:
Top Health Industry Issue of 2018
PwC Health Research Institute Top Health Industry Issues of 2018 Report: Issuu Slide Presentation
12 Defining Healthcare Issues of 2018
Is Laboratory Medicine Ready for Artificial Intelligence?
Artificial Intelligence Imaging Research Facilitates Disease Diagnosis
Blockchain Technology Could Impact How Clinical Laboratories and Pathology Groups Exchange Lab Test Data
Skepticism, Distrust of HIT by Healthcare Consumers Undermines Physician Adoption of Medical Reporting Technologies, But Is Opportunity for Pathology Groups, Clinical Laboratories
Pathologists and Clinical Lab Executives Take Note: Medicare Has New Goals and Deadlines for Transitioning from Fee-For-Service Healthcare Models to Value-Based Reimbursement
Researchers Point to Cost of Services, including Medical Laboratories, for Healthcare Spending Gap Between the US and Other Developed Countries
Telemedicine and Microhospitals Could Make Up for Reducing Numbers of Primary Care Physicians in US Urban and Metro Suburban Areas
New FDA Regulations of Clinical Decision-Support/Digital Health Applications and Medical Software Has Consequences for Medical Laboratories
Lab-on-a-Fiber Technology Continues to Highlight Nano-Scale Clinical Laboratory Diagnostic Testing in Point-of-Care Environments
Johns Hopkins’ Test Drone Travels 161 Miles to Set Record for Delivery Distance of Clinical Laboratory Specimens
Apr 2, 2018 | Digital Pathology, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing
Ever shrinking “lab-on-a-…” technologies, a boon to medical laboratories and anatomic pathologists in remote resource-strapped regions, also have a place in modern labs
Researchers took another leap forward in reducing the size of clinical laboratory diagnostic tests and observational tools. This demonstration involved lab-on-a-fiber technology and showed promise in both monitoring anatomic pathology biomarkers in vivo and supplementing the abilities of existing lab-on-a-chip and microfluidic devices.
Lab-on-a-Fiber Next Technological Step Toward Miniaturization
In 2013, Dark Daily reported on research into an implantable laboratory-on-a-chip (LOC) for monitoring blood chemistry during chemotherapy. It was a major breakthrough at the time, which promised new and powerful tools for cancer treatment regimens.
However, most LOC systems aren’t designed for wet environments. Also, while microfluidics and flexible membranes allow for smaller footprints and tighter placement, they are still invasive in ways that might make patients uncomfortable or make real-world use less than ideal. And, long-term use brings further complications, such as corrosion or foreign-body granulomas.
Thus, lab-on-a-fiber’s ability to function in vivo, is one of the device’s principal advantages, as ExtremeTech noted.
Lab-on-a-fiber technology addresses many concerns. It is small enough to insert directly into organs, muscle mass, or veins when used as biosensors. And the fibers can return a wealth of information by using light and reflection, while allowing for minimal discomfort and precision placement.
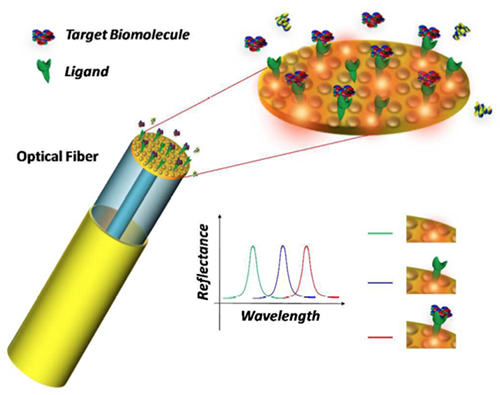
Schematic of the lab-on-a-fiber biosensing principle. A metallic nanostructure supporting a resonant plasmonic mode is integrated on the optical fiber tip. When a molecular binding event occurs at the sensor surface, the reflectance peak associated to the plasmonic mode shifts towards longer wavelengths. (Image and caption copyright: Analyst/The Royal Society of Chemistry.)
The Past and Future of Scaling Clinical Laboratory Testing
Dark Daily has followed these miniaturization trends for years starting with their earliest stages. A detailed timeline of developments can be viewed in “Lab-on-a-Chip Diagnostics: When Will Clinical Laboratories See the Revolution?” from 2016.
Additional Dark Daily “lab-on-a-…” coverage includes:
- IBM Watson Health and Mount Sinai Health System team up to use LOC solutions to separate biomolecules as small as 20nm from samples (See Dark Daily, “IBM and Mount Sinai Researchers Develop Innovative Medical Lab-on-a-Chip Solution,” October 3, 2016); and,
- Lab-on-Skin devices for monitoring biomarkers and electrophysiological signals, providing human-machine interfaces, and facilitating optogenetics (See Dark Daily, “In the Field of Nano-Scale Diagnostics, Many Researchers Are Developing ‘Lab-on-Skin’ Technologies That Can Monitor Many Clinical Laboratory Biomarkers,” January 15, 2015).
In the past year, a myriad of lab-on-a-fiber applications also have received media coverage, including:
Developers believe lab-on-a-fiber approaches could offer further adaptability and functionality to other “lab-on-a-…” technologies. For example, as highlighted in Advanced Science News, researchers are employing lab-on-a-fiber technologies to further refine and improve LOC functions and designs.
“As the scientific world moves inexorably to smaller dimensions … The emerging concept of ‘lab‐on‐fiber’ will give the optical fiber platform additional (highly integrated) functionalities,” noted Deepak Uttamchandani, PhD, Vice Dean Research, Faculty of Engineering, and, Robert Blue, PhD, Research Fellow, both at the University of Strathclyde, Glasgow, UK, in their review paper, “Recent Advances In Optical Fiber Devices for Microfluidics Integration.” The paper, published in the Journal of Biophotonics, examined “the recent emergence of miniaturized optical fiber-based sensing and actuating devices that have been successfully integrated into fluidic microchannels that are part of microfluidic and lab‐on‐chip systems.”

In his review paper on the emerging concept of lab-on-a-fiber, Deepak Uttamchandani, PhD, notes, “The versatility of the optical fiber platform has already allowed researchers to conduct immunoassays in microchannels using both fluorescently‐labelled and label‐free formats whilst gaining advantages of reduced assay time and increased sensitivity.” (Photo copyright: University of Strathclyde.)
Lab-on-a-Fiber: Another Step Forward or a Major Change?
At each milestone in the scaling of clinical laboratory testing, experts and media outlets predicted the demise of big laboratories and the dawn of a POC-centric testing era. Yet, despite 20-plus years of progress, this has yet to happen.
While it is critical for anatomical pathology leaders and clinical laboratory managers to stay abreast of developments in testing technology, much of the innovation behind lab-on-a-fiber remains strictly in the research realm. Challenges to the commercialization of these new techniques include both physical factors, such as design and manufacture of ready-to-use tests, and regulatory concerns, including FDA clearances and payer approval of new assays and diagnostic procedures.
Until researchers and test manufacturers overcome these hurdles, threats to current standards and workflows are minimal. However, much like the gains in scale realized through incorporating lab-on-a-chip concepts into clinical laboratory testing, the research powering these innovations might prove useful in further improving and expanding medical laboratory testing options.
—Jon Stone
Related Information:
Optical Fiber Devices for Microfluidics Integration Open Up New Horizons for Advanced “Lab-on-a-Chip” Technologies
Recent Advances in Optical Fiber Devices for Microfluidics Integration
Lab-on-Fiber Technology: A New Vision for Chemical and Biological Sensing [Abstract]
Lab-on-Fiber Technology: A New Vision for Chemical and Biological Sensing [Full Downloadable PDF]
How We’re Shrinking Chemical Labs onto Optical Fibers
Lab-on-Fiber Could Shine Light on Disease
Doctors Might Soon Diagnose You by Feeding a Lab-on-a-Fiber Straight into Your Veins
Fiber-Optic Device Can Detect Stray Cancer Cells and Improve Tumor Removal: Study
Fiber Optic Probe Beats a Biopsy for Measuring Muscle Health
Lab-on-a-Chip Diagnostics: When Will Clinical Laboratories See the Revolution?
Implantable Medical Laboratory-on-a-Chip Continuously Monitors Key Chemicals in Chemotherapy and High-Risk Patients
In the Field of Nano-Scale Diagnostics, Many Researchers Are Developing ‘Lab-on-Skin’ Technologies That Can Monitor Many Clinical Laboratory Biomarkers
Hematology on a Chip: University of Southampton Develops POC Blood Analysis
Sleek ‘Lab in a Needle’ Is an All-in-One Device That Detects Liver Toxicity in Minutes during a Study, Showing Potential to Supplant Some Medical Laboratory Tests
Whole Animal Assays Use Lab-on-a-Chip at MIT
IBM and Mount Sinai Researchers Develop Innovative Medical Lab-on-a-Chip Solution
In the Field of Nano-Scale Diagnostics, Many Researchers Are Developing ‘Lab-on-Skin’ Technologies That Can Monitor Many Clinical Laboratory Biomarkers
Mar 31, 2017 | Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology
This year, one of IBM’s closely-watched picks of the technologies most likely to have the greatest impact on society is the medical lab-on-a-chip
Clinical laboratory testing and diagnostics are one of the five technologies included in IBM’s 2017 list of the technologies it predicts will have the greatest impact on society during the next five years. Of equal interest to medical laboratory professionals is that several of the other technologies included in IBM’s list have the potential be used in medical laboratories and anatomic pathology groups.
IBM Research, corporate research laboratory for parent company IBM (NYSE:IBM), has more than 3,000 researchers working in 12 labs on six continents. Each year the lab releases a list of five technologies it forecasts will have the greatest influence on how our bodies, minds, society, and the planet, develop over the next five years. The list is called “5-in-5” and has been released annually for the past 10 years by the tech giant. (more…)
Oct 3, 2016 | Digital Pathology, Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing
Clinical laboratories and pathology groups may eventually use these devices to detect minute quantities of biomarkers
IBM has regularly declared its interest in being a player in the field of healthcare big data. Now comes news that the information technology giant wants to develop lab-on-a-chip (LOC) technology that can handle different types of clinical laboratory and anatomic pathology tests.
As reported in Nature Nanotechnology, researchers at IBM are working with a team from Mount Sinai Health System. Together, they created a lab-on-a-chip device capable of separating biomolecules as small as 20nm in length from urine, saliva, or blood samples without the need for specialized clinical laboratory equipment. The technology is called nanoDLD.
Current testing of this lab-on-a-chip focuses on exosomes and cancer research. However, researchers note that the asymmetric pillar array on their silicon chip can also separate DNA, viruses, and protein complexes. With further development, they hope to separate particles down to 10nm in length. This would allow isolation of specific proteins. (more…)






